Foamable pluroleosomes system loaded with amlodipine as a repurposed antibacterial topical formulation against MRSA-induced infection; optimization, in-vitro, ex-vivo, and in-vivo studies
IF 6.4
2区 医学
Q1 PHARMACOLOGY & PHARMACY
引用次数: 0
Abstract
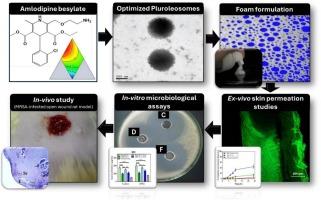
Amlodipine besylate (AML) is a renowned antihypertensive drug currently acknowledged for having antibacterial activity. AML repositioning can be helpful in the defeat of microbial-resistant strains. Loading amlodipine in the pluroleosomes (PLOs) foam system is desired to approach innovative remedies with a convenient application capable of targeting deep infections. The mixture design was employed to generate different pluroleosomes formulations consisting of various ratios of Pluronic F-127, oleic acid, and soya lecithin loaded with amlodipine. Based on the desirability function, the selected optimized formula (AML-PLOs), consisting of 4.875 for lecithin, one for oleic acid, and 1.125 for pluronic, exhibits a particle size of 320.56 ± 15.5 nm, a polydispersity index of 0.4461 ± 0.03, a surface charge of 15.261 ± 0.62 mV, and AML entrapment of 71.25 ± 3.52 %. The morphological image displayed a uniform spherical shape at the nanoscale. In addition, thermal analysis and infrared spectroscopy (IR) proved the suitability of AML-pluroleosome vesicles. Tween 20, the selected nonionic surfactant in foam preparation, achieved the demand values of foam parameters and showed adequate stability upon storage for up to 90 days. The selected AML-PLO foam showed complete AML release after 48 h in a controlled manner, and the cumulative amount permeated after 24 h was about 45 %. Efficient penetration through dermal strata was affirmed by utilizing a confocal microscope. In vitro microbiological assay, besides the in vivo microbiological and histopathological studies employing a wound healing model, validated the antibacterial efficacy of amlodipine. Those outcomes demonstrated that the prepared pluroleosome foam system of AML is a competent candidate for combating topical bacterial infection.
负载氨氯地平的泡沫多聚体系统作为抗mrsa诱导感染的重新用途抗菌外用制剂优化,体外,离体和体内研究
苯磺酸氨氯地平(AML)是一种著名的抗高血压药物,目前公认具有抗菌活性。AML重新定位可能有助于击败微生物耐药菌株。在多聚体(PLOs)泡沫系统中加载氨氯地平是一种创新的疗法,具有方便的应用能力,能够靶向深度感染。采用混合设计制备了由不同比例的Pluronic F-127、油酸和大豆卵磷脂负载氨氯地平组成的多聚体配方。优选出的优化配方(AML- plos)为卵磷脂(4.875)、油酸(1)、pluronic(1.125),粒径为320.56±15.5 nm,多分散性指数为0.4461±0.03,表面电荷为15.261±0.62 mV, AML包载率为71.25±3.52%。形态学图像在纳米尺度上呈现均匀的球形。此外,热分析和红外光谱(IR)证实了aml -多聚体囊泡的适用性。泡沫制备中选用的非离子表面活性剂Tween - 20达到了泡沫参数的要求值,并在长达90天的储存中表现出足够的稳定性。所选择的AML- plo泡沫在48 h后可控制地完全释放AML, 24 h后的累积渗透量约为45%。利用共聚焦显微镜确认有效穿透真皮层。体外微生物学分析以及采用伤口愈合模型的体内微生物学和组织病理学研究验证了氨氯地平的抗菌效果。这些结果表明,制备的AML多聚体泡沫体系是对抗局部细菌感染的有效候选物。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

International Journal of Pharmaceutics: X
Pharmacology, Toxicology and Pharmaceutics-Pharmaceutical Science
CiteScore
6.60
自引率
0.00%
发文量
32
审稿时长
24 days
期刊介绍:
International Journal of Pharmaceutics: X offers authors with high-quality research who want to publish in a gold open access journal the opportunity to make their work immediately, permanently, and freely accessible.
International Journal of Pharmaceutics: X authors will pay an article publishing charge (APC), have a choice of license options, and retain copyright. Please check the APC here. The journal is indexed in SCOPUS, PUBMED, PMC and DOAJ.
The International Journal of Pharmaceutics is the second most cited journal in the "Pharmacy & Pharmacology" category out of 358 journals, being the true home for pharmaceutical scientists concerned with the physical, chemical and biological properties of devices and delivery systems for drugs, vaccines and biologicals, including their design, manufacture and evaluation. This includes evaluation of the properties of drugs, excipients such as surfactants and polymers and novel materials. The journal has special sections on pharmaceutical nanotechnology and personalized medicines, and publishes research papers, reviews, commentaries and letters to the editor as well as special issues.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


