A self-delivery albumin nanomedicine amplified photodynamic therapy against esophageal cancer through COX-2/PGE2 interruption and regulation of mitochondrial respiratory
IF 6.4
2区 医学
Q1 PHARMACOLOGY & PHARMACY
引用次数: 0
Abstract
Photodynamic therapy (PDT) has emerged as a promising non-invasive cancer treatment due to its selective tumor ablation and excellent safety characteristics. However, its efficacy is limited by tumor hypoxia and excessive inflammation. In this study, we fabricated human serum albumin-based nanoparticles (CAI NPs) encapsulating celecoxib (CXB), atovaquone (ATO), and IR820 via sonication. The CAI NPs exhibited favorable physicochemical properties, including a uniform size distribution (<200 nm), high encapsulation efficiency and excellent colloidal stability. Initially, ATO acts as a mitochondrial complex III inhibitor, suppressing oxidative phosphorylation to ameliorate tumor hypoxia. This hypoxia alleviation potentiates PDT efficacy by enhancing tumor cell ROS generation. Furthermore, concomitant COX-2/PGE2 inhibition by CXB attenuates the excessive inflammatory cascade triggered during PDT, resulting in enhanced therapeutic outcomes through microenvironment modulation. Eventually, the dual-enhanced CAI NPs demonstrate potent antitumor activity in both in vivo and ex vivo models, while maintaining excellent biocompatibility under physiological conditions. In summary, the integrated three-drug regimen conclusively enhances photodynamic therapeutic outcomes through multimodal mechanisms, establishing a viable treatment approach for esophageal cancer.
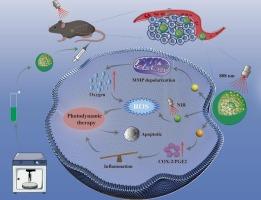
自释白蛋白纳米药物通过阻断COX-2/PGE2和调节线粒体呼吸增强光动力治疗食管癌
光动力疗法(PDT)因其选择性消融肿瘤和良好的安全性而成为一种很有前途的非侵入性癌症治疗方法。但其疗效受肿瘤缺氧和过度炎症的限制。在这项研究中,我们通过超声制备了基于人血清白蛋白的纳米颗粒(CAI NPs),该纳米颗粒包被塞来昔布(CXB)、阿托伐醌(ATO)和IR820。CAI纳米粒子具有良好的物理化学性质,包括粒径分布均匀(约200 nm)、包封效率高和胶体稳定性好。最初,ATO作为线粒体复合物III抑制剂,抑制氧化磷酸化以改善肿瘤缺氧。这种缺氧缓解通过增强肿瘤细胞ROS的产生来增强PDT的疗效。此外,CXB同时抑制COX-2/PGE2可减轻PDT期间引发的过度炎症级联反应,通过微环境调节提高治疗效果。最终,双增强CAI NPs在体内和离体模型中都显示出强大的抗肿瘤活性,同时在生理条件下保持良好的生物相容性。综上所述,三药联合方案通过多模式机制提高了光动力治疗效果,为食管癌的治疗建立了可行的方法。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

International Journal of Pharmaceutics: X
Pharmacology, Toxicology and Pharmaceutics-Pharmaceutical Science
CiteScore
6.60
自引率
0.00%
发文量
32
审稿时长
24 days
期刊介绍:
International Journal of Pharmaceutics: X offers authors with high-quality research who want to publish in a gold open access journal the opportunity to make their work immediately, permanently, and freely accessible.
International Journal of Pharmaceutics: X authors will pay an article publishing charge (APC), have a choice of license options, and retain copyright. Please check the APC here. The journal is indexed in SCOPUS, PUBMED, PMC and DOAJ.
The International Journal of Pharmaceutics is the second most cited journal in the "Pharmacy & Pharmacology" category out of 358 journals, being the true home for pharmaceutical scientists concerned with the physical, chemical and biological properties of devices and delivery systems for drugs, vaccines and biologicals, including their design, manufacture and evaluation. This includes evaluation of the properties of drugs, excipients such as surfactants and polymers and novel materials. The journal has special sections on pharmaceutical nanotechnology and personalized medicines, and publishes research papers, reviews, commentaries and letters to the editor as well as special issues.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


