Valorization of chitosan-derived humins for removal of Cd(II), Cr(III) and Cr(VI)
IF 7.1
Q1 ENGINEERING, CHEMICAL
引用次数: 0
Abstract
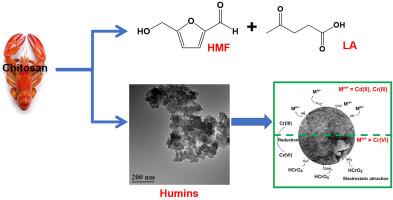
The generation of humins by-products in biorefinery is often unavoidable. We here report the valorization of chitosan-derived humins for removal of heavy metal ions to enhance the atomic economy of the biomass conversion. Chitosan-derived humins were synthesized by catalysis of oxalic acid (OA), whereby the adsorption performance for Cd(II), Cr(III) and Cr(VI) was explored. The adsorption data were well fitted by the pseudo-second-order kinetic model, complemented by the Freundlich and Temkin isotherm models, revealing maximum adsorption capacities of humins for heavy metal ions as follows: 358.7 mg/g for Cr(VI), 152.6 mg/g for Cd(II), and 40.2 mg/g for Cr(III). The mechanistic studies revealed that the adsorption of Cd(II) and Cr(III) by humins mainly occurs through ion exchange (carboxyl groups) and surface complexation (hydroxyl, carbonyl, and amino groups). In comparison, the adsorption of Cr(VI) by humins involves electrostatic attraction, alongside ion exchange and surface complexation. After carbonization and activation, the specific surface area increased markedly, however the adsorption capacity decreased owing to reduced oxygen- and nitrogen-containing functional groups. Competitive adsorption studies, industrial wastewater experiments, and cyclic adsorption tests demonstrate that humins exhibit remarkable adsorption performance even in complex environments. This work highlights that chitosan-derived humins are promising adsorbents for the remediation of heavy metal ions.
壳聚糖衍生的人蛋白对Cd(II)、Cr(III)和Cr(VI)的吸附性能研究
生物炼制过程中产生的人类副产品往往是不可避免的。我们在此报道壳聚糖衍生的人类毒素用于去除重金属离子,以提高生物质转化的原子经济性。以草酸(OA)为催化剂合成壳聚糖衍生的人类素,考察其对Cd(II)、Cr(III)和Cr(VI)的吸附性能。拟二级动力学模型对吸附数据拟合良好,Freundlich和Temkin等温线模型对吸附数据进行了补充,结果表明人体对重金属离子的最大吸附量为:Cr(VI) 358.7 mg/g, Cd(II) 152.6 mg/g, Cr(III) 40.2 mg/g。机理研究表明,人素对Cd(II)和Cr(III)的吸附主要通过离子交换(羧基)和表面络合(羟基、羰基和氨基)进行。相比之下,人类对Cr(VI)的吸附包括静电吸引、离子交换和表面络合。炭化活化后,比表面积明显增加,但由于含氧和含氮官能团的减少,吸附能力下降。竞争吸附研究、工业废水实验和循环吸附试验表明,即使在复杂的环境中,人类也表现出卓越的吸附性能。这项工作强调了壳聚糖衍生的人类素是一种有前途的重金属离子修复吸附剂。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Chemical Engineering Journal Advances
Engineering-Industrial and Manufacturing Engineering
CiteScore
8.30
自引率
0.00%
发文量
213
审稿时长
26 days
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


