Integrated transcriptomic and metabolomic analyses reveal the potential mechanisms underlying sex- and size-dependent resistance to Vibrio alginolyticus infection in the Chinese mitten crab (Eriocheir sinensis)
IF 3.9
1区 农林科学
Q1 FISHERIES
引用次数: 0
Abstract
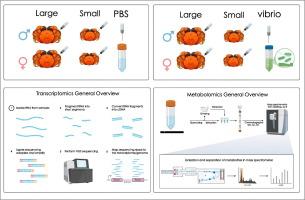
In this study, we performed an integrative analysis of transcriptomic and metabolomic data to systematically investigate the influence of two key phenotypic factors—sex and body size—on the resistance of Eriocheir sinensis (Chinese mitten crab) to Vibrio alginolyticus infection. Our findings revealed that sex exerts a substantially greater impact on bacterial resistance than body size. Among the four phenotypic groups, large-sized females exhibited the strongest resistance, followed by small-sized females, large-sized males, and small-sized males, indicating a clear trend of “females > males” and “large size > small size” in resistance capacity.
Multi-omics analyses further uncovered the molecular basis underlying these phenotypic differences. At the transcriptomic level, female crabs exhibited significantly higher activation of immune-related pathways, including endocytosis, immune signaling, cytoskeletal regulation, and apoptosis, suggesting a stronger innate immune response compared to males. At the metabolomic level, large-sized individuals showed enhanced energy metabolic pathways, potentially providing the energetic support required to sustain robust immune responses.Moreover, this study is the first to comprehensively characterize the endocytic pathway utilized by E. sinensis in response to Vibrio challenge, including pathogen recognition and endocytic initiation, endosomal transport and maturation, and lysosomal degradation. Notably, the regulation of endocytic immune responses was also found to be more strongly influenced by sex than by body size.
In summary, this study provides a systems-level understanding of how sex and body size collaboratively modulate the immune resistance of E. sinensis to Vibrio infection, offering valuable insights and molecular markers for disease-resistant breeding and precision aquaculture management in crustaceans.
整合转录组学和代谢组学分析揭示了中华绒螯蟹(Eriocheir sinensis)对溶藻弧菌感染的性别和大小依赖性抗性的潜在机制
在这项研究中,我们对转录组学和代谢组学数据进行了综合分析,系统地研究了两个关键表型因素-性别和体型-对中华绒螯蟹对溶藻弧菌感染的抗性的影响。我们的研究结果表明,性别对细菌耐药性的影响要比体型大得多。在4个表型组中,大体型雌蚊的抗性最强,其次是小体型雌蚊、大体型雄蚊和小体型雄蚊,抗性能力呈现明显的“雌性&雄性”和“大体型&小体型”的趋势。多组学分析进一步揭示了这些表型差异背后的分子基础。在转录组水平上,雌蟹表现出明显更高的免疫相关途径的激活,包括内吞作用、免疫信号传导、细胞骨架调节和凋亡,表明与雄蟹相比,雌蟹具有更强的先天免疫应答。在代谢组学水平上,体型较大的个体表现出增强的能量代谢途径,可能提供维持强大免疫反应所需的能量支持。此外,本研究首次全面表征了中华梭菌在应对弧菌挑战时所利用的内吞途径,包括病原体识别和内吞起始、内体运输和成熟以及溶酶体降解。值得注意的是,内吞免疫反应的调节也被发现受到性别比体型更强烈的影响。综上所述,本研究在系统水平上了解了性别和体型如何协同调节中华赤霉素对弧菌感染的免疫抗性,为甲壳类动物的抗病育种和精准养殖管理提供了有价值的见解和分子标记。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Aquaculture
农林科学-海洋与淡水生物学
CiteScore
8.60
自引率
17.80%
发文量
1246
审稿时长
56 days
期刊介绍:
Aquaculture is an international journal for the exploration, improvement and management of all freshwater and marine food resources. It publishes novel and innovative research of world-wide interest on farming of aquatic organisms, which includes finfish, mollusks, crustaceans and aquatic plants for human consumption. Research on ornamentals is not a focus of the Journal. Aquaculture only publishes papers with a clear relevance to improving aquaculture practices or a potential application.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


