NH2@MoS2 impregnated MXene adsorbents for eliminating cationic dyes: Insight into pH, ionic strength and adsorption mechanism
IF 5.1
3区 材料科学
Q2 MATERIALS SCIENCE, COATINGS & FILMS
引用次数: 0
Abstract
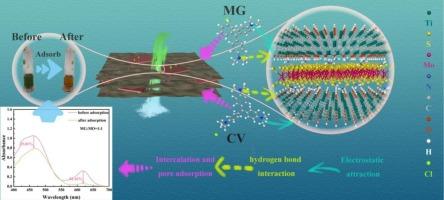
Herein, MXene/NH2@MoS2 adsorbent (MXMo-x) were facilely synthesized by introducing 3-aminopropyltriethoxysilane (APTES)-modified molybdenum disulfide (NH2@MoS2) into a MXene matrix through hydrothermal self-assembly and freeze-drying process. The abundant functional groups of NH2@MoS2 coupled with unique layer structure prevents the agglomeration of MXene, facilitates hierarchical porous structures formation, and promotes the specific surface area from 2.65 m2/g (MXene) to 8.27 m2/g (MXMo-6). This eventually leads to ultrahigh maximum adsorption capacities of 1170 mg/g for Malachite Green (MG) and 1050 mg/g for Crystal Violet (CV) at pH 10 due to the synergetic effect of electrostatic attraction, hydrogen bonding and pore filling. Particularly, the adsorption of MG and CV exhibited an insignificant decrease with the concentration of salt solutions (Na+, Mg2+), and the adsorption capacity of MG/CV in tap water and lake water also reached up to 961/867 mg/g and 842/732 mg/g, showing good salinity resistance and potential applicability in complex water systems. After five adsorption-desorption cycles, the adsorption capacity decreased by only 10 %, proving good recyclability of the adsorbents. Studies show that the adsorption process is a spontaneous endothermic process, which conforms to the quasi-second-order kinetic model (R2 > 0.99) and the Freundlich isothermal adsorption model (R2 > 0.91), revealing the process is primarily controlled by chemisorptions mechanisms. This study provides theoretical insights and technical references for the design of MXene-based composites and their application in dye wastewater treatment.
NH2@MoS2浸渍MXene吸附剂去除阳离子染料:洞察pH,离子强度和吸附机理
本文将3-氨基丙基三乙氧基硅烷(APTES)修饰的二硫化钼(NH2@MoS2)引入MXene基体,经水热自组装和冷冻干燥工艺,制备了MXene/NH2@MoS2吸附剂(MXMo-x)。NH2@MoS2丰富的官能团加上独特的层状结构,阻止了MXene的团聚,有利于分层多孔结构的形成,使比表面积从2.65 m2/g (MXene)提高到8.27 m2/g (MXMo-6)。在pH值为10时,孔雀石绿(mg)和结晶紫(CV)的最大吸附量分别为1170 mg/g和1050 mg/g,这是由于静电吸引、氢键和孔隙填充的协同作用。MG和CV的吸附量随盐溶液(Na+、Mg2+)浓度的增加而显著降低,MG/CV在自来水和湖水中的吸附量分别达到961/867 MG/ g和842/732 MG/ g,具有良好的耐盐性和在复杂水系中的潜在适用性。经过5次吸附-解吸循环后,吸附量仅下降10%,表明吸附剂具有良好的可回收性。研究表明,吸附过程为自发吸热过程,符合准二级动力学模型(R2 > 0.99)和Freundlich等温吸附模型(R2 > 0.91),表明吸附过程主要受化学吸附机理控制。本研究为mxene基复合材料的设计及其在染料废水处理中的应用提供了理论见解和技术参考。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Diamond and Related Materials
工程技术-材料科学:综合
CiteScore
6.00
自引率
14.60%
发文量
702
审稿时长
2.1 months
期刊介绍:
DRM is a leading international journal that publishes new fundamental and applied research on all forms of diamond, the integration of diamond with other advanced materials and development of technologies exploiting diamond. The synthesis, characterization and processing of single crystal diamond, polycrystalline films, nanodiamond powders and heterostructures with other advanced materials are encouraged topics for technical and review articles. In addition to diamond, the journal publishes manuscripts on the synthesis, characterization and application of other related materials including diamond-like carbons, carbon nanotubes, graphene, and boron and carbon nitrides. Articles are sought on the chemical functionalization of diamond and related materials as well as their use in electrochemistry, energy storage and conversion, chemical and biological sensing, imaging, thermal management, photonic and quantum applications, electron emission and electronic devices.
The International Conference on Diamond and Carbon Materials has evolved into the largest and most well attended forum in the field of diamond, providing a forum to showcase the latest results in the science and technology of diamond and other carbon materials such as carbon nanotubes, graphene, and diamond-like carbon. Run annually in association with Diamond and Related Materials the conference provides junior and established researchers the opportunity to exchange the latest results ranging from fundamental physical and chemical concepts to applied research focusing on the next generation carbon-based devices.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


