Synthesis, spectral characterization, Z-scan and NLO detection sensitivity and applications of various DFT calculation methods on a new Zn(II) complex
IF 5.4
3区 化学
Q1 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
Abstract
The synthesized Zn(II) complex of 3−/6-methylpicolinic acid (3−/6-MepicH) was structurally characterized by mass spectrometry (MS) as well as 1H and 13C NMR spectroscopy. The spectral features were investigated by the FTIR and UV–Vis spectra. To elucidate third−order nonlinear optical (NLO) characteristic, Z-scan study was fulfilled. Theoretical characterizations were carried out using different featured DFT methods. Moreover, the values of external electric field (E), polarization (P), and electric displacement (D) for the Zn(II) complex were computed employing DFT/M06-L, CAM-B3LYP, and HSEh1PBE functionals. Similarly, the linear optical (LO) susceptibility and polarization parameters χ(1)/P(1) as well as the second- and third-order nonlinear optical (NLO) tensors χ(2)/P(2) and χ(3)/P(3) were derived at the same computational levels. Furthermore, the refractive index (n) and optical band gap were determined within the UV–Vis spectrum. Subsequently, the Zn(II) complex's static and dynamic LO and NLO properties were examined at the DFT/M06-L, CAM-B3LYP, and HSEh1PBE levels. The electronic band gap (Eg), dipole moment and polarizability values were obtained at 4.37 eV (experimental) and 4.777 eV (DFT/M06-L), 8.009 D (DFT/M06-L), <α(0,0)> 43.134 × 10−24 esu and <α(−ω;ω)> 38.954 × 10−24 esu, respectively. From the Z-scan experiments, the complex exhibited a third-order nonlinear optical susceptibility (χ(3)) of 62.5068 × 10−4 and a second-order molecular hyperpolarizability (γ) of −6532.41 × 10−28 esu. Using the DFT/M06-L method, the values of <γ(0,0,0,0)>/<γ(−ω;ω,0,0)> and <γ(−2ω;ω,ω,0) > third-order nonlinear optical susceptibilities of the complex in ethanol were determined as 70.427 × 10−36, 94.085 × 10−36 and 2599.27 × 10−36 esu, respectively. The significant nonlinear optical responses from Z-scan measurements and DFT-based calculations reveal that the compound exhibits promising characteristics for advanced optical sensing applications, including intensity-dependent detectors and environmentally responsive phase-modulating sensors.
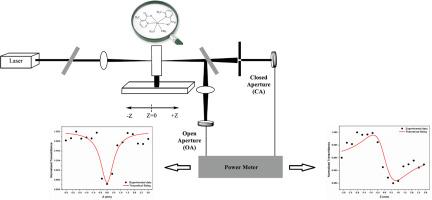
一种新型Zn(II)配合物的合成、光谱表征、z扫描和NLO检测灵敏度及各种DFT计算方法的应用
合成的3−/6-甲基吡啶酸Zn(II)配合物(3−/6-MepicH)通过质谱(MS)、1H和13C NMR进行了结构表征。用红外光谱和紫外可见光谱研究了其光谱特征。为了阐明三阶非线性光学(NLO)特性,进行了z扫描研究。采用不同的特征DFT方法进行了理论表征。利用DFT/M06-L、CAM-B3LYP和HSEh1PBE等官能团计算了Zn(II)配合物的外电场(E)、极化(P)和电位移(D)值。同样,在相同的计算水平上导出了线性光学(LO)磁化率和偏振参数χ(1)/P(1)以及二阶和三阶非线性光学(NLO)张量χ(2)/P(2)和χ(3)/P(3)。在紫外可见光谱范围内测定了折射率(n)和光学带隙。随后,在DFT/M06-L、CAM-B3LYP和HSEh1PBE水平下检测Zn(II)配合物的静态和动态LO和NLO性质。分别在4.37 eV(实验)和4.777 eV (DFT/M06-L)、8.009 D (DFT/M06-L)、<α(0,0)>、43.134 × 10−24 esu和<;α(−ω;ω)>、38.954 × 10−24 esu下得到电子带隙(Eg)、偶极矩和极化率值。z扫描实验表明,配合物的三阶非线性光学磁化率(χ(3))为62.5068 × 10−4,二阶分子超极化率(γ)为- 6532.41 × 10−28 esu。利用DFT/M06-L方法,测定了配合物在乙醇中的三阶非线性光学敏感度<;γ(−ω;ω,0,0)> /<γ(−ω;ω,0,0)>;和<;γ(−2ω;ω,ω,0) >;分别为70.427 × 10−36、94.085 × 10−36和2599.27 × 10−36 esu。z -扫描测量和基于dft的计算的显著非线性光学响应表明,该化合物具有先进的光学传感应用前景,包括强度相关探测器和环境响应相位调制传感器。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Inorganic Chemistry Communications
化学-无机化学与核化学
CiteScore
5.50
自引率
7.90%
发文量
1013
审稿时长
53 days
期刊介绍:
Launched in January 1998, Inorganic Chemistry Communications is an international journal dedicated to the rapid publication of short communications in the major areas of inorganic, organometallic and supramolecular chemistry. Topics include synthetic and reaction chemistry, kinetics and mechanisms of reactions, bioinorganic chemistry, photochemistry and the use of metal and organometallic compounds in stoichiometric and catalytic synthesis or organic compounds.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


