Selective leaching of arsenic from zinc oxide dust in NaOH-(NH2)2CS solution
IF 4.8
2区 材料科学
Q1 METALLURGY & METALLURGICAL ENGINEERING
引用次数: 0
Abstract
The selective removal of arsenic from zinc smelting dust is a notable measure toward resource recovery and environmental protection. In this study, a thiourea-assisted alkaline leaching method was employed to selectively extract arsenic from Zn smelting dust. The thermodynamics of arsenic dissolution were investigated first. The effects of NaOH concentration, thiourea concentration, leaching temperature, leaching time, and liquid–solid ratio on arsenic and zinc extraction during alkaline leaching were investigated. The results demonstrated that the addition of thiourea effectively enhanced arsenic extraction from 58.1 % to 77.1 %, while suppressing Zn leaching from 18.4 % to 2.6 %. Under optimal conditions, As, Zn, Fe, Pb, In, and Ag exhibited leaching efficiencies of 77.1 %, 2.6 %, 4.5 %, 2.1 %, 1.3 %, and 3.2 %, respectively, confirming selective arsenic leaching over other elements. Combined XRD, SEM, and XPS characterizations revealed that thiourea facilitated the dissolution of insoluble arsenate minerals and promoted the formation of ZnS and PbS precipitates by releasing S2− into the solution. This process effectively reduced the loss of valuable metals such as Zn and Pb. This method can be used to selectively remove arsenic from As-containing oxidized dust.
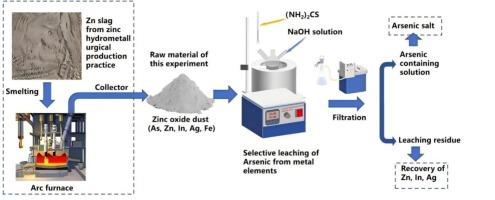
NaOH-(NH2)2CS溶液中氧化锌粉尘中砷的选择性浸出
锌冶炼粉尘中砷的选择性脱除是资源回收和环境保护的重要措施。采用硫脲辅助碱性浸出法从锌冶炼粉尘中选择性提取砷。首先研究了砷溶解的热力学。考察了碱浸过程中NaOH浓度、硫脲浓度、浸出温度、浸出时间和液固比对砷锌浸出的影响。结果表明,硫脲的加入可有效地将砷的浸出率从58.1%提高到77.1%,同时抑制锌的浸出率从18.4%降低到2.6%。在最佳条件下,As、Zn、Fe、Pb、In和Ag的浸出效率分别为77.1%、2.6%、4.5%、2.1%、1.3%和3.2%,证实了砷对其他元素的选择性浸出。XRD、SEM和XPS表征表明,硫脲通过向溶液中释放S2−,促进了不溶性砷酸盐矿物的溶解,促进了ZnS和PbS沉淀的形成。该工艺有效地减少了锌、铅等贵重金属的损失。该方法可用于选择性地去除含砷氧化粉尘中的砷。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Hydrometallurgy
工程技术-冶金工程
CiteScore
9.50
自引率
6.40%
发文量
144
审稿时长
3.4 months
期刊介绍:
Hydrometallurgy aims to compile studies on novel processes, process design, chemistry, modelling, control, economics and interfaces between unit operations, and to provide a forum for discussions on case histories and operational difficulties.
Topics covered include: leaching of metal values by chemical reagents or bacterial action at ambient or elevated pressures and temperatures; separation of solids from leach liquors; removal of impurities and recovery of metal values by precipitation, ion exchange, solvent extraction, gaseous reduction, cementation, electro-winning and electro-refining; pre-treatment of ores by roasting or chemical treatments such as halogenation or reduction; recycling of reagents and treatment of effluents.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


