Enhancing electrochromism and photoluminescence in carbazole-viologens via conjugation extension and dimerization suppression
IF 4.2
3区 工程技术
Q2 CHEMISTRY, APPLIED
引用次数: 0
Abstract
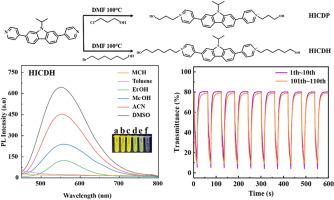
Viologens exhibit reversible redox properties and remarkable color response, making them core materials in electrochromism. However, their low conjugation compromises charge transfer efficiency, undermines redox reversibility, and causes fluorescence quenching, thereby limiting their applications. This study addresses this challenge by proposing a synergistic design strategy of 'aromatic bridging–flexible side chains.' Incorporating electron-rich carbazole groups into the viologen backbone as a rigid bridging structure expands the molecular conjugation network; while tuning the length of the alkyl side chains on both sides suppresses molecular aggregation. Following this strategy, we designed and synthesized carbazole-viologen derivatives substituted with hydroxypropyl (HICDP) and hydroxyhexyl (HICDH) groups. A systematic investigation revealed how the carbazole unit and side-chain length regulate their electrochromic and photoluminescent properties. Experiments showed that the carbazole bridging unit expanded the conjugation system, enabling HICDH to achieve an optical contrast of ΔT = 77.4 % at 700 nm. Even after 100 cycles, HICDH retained approximately 70 % of its optical contrast. The increased side-chain length in carbazole-viologen derivatives increased the distance between radicals, suppressed dimer formation, and enhanced stability. Meanwhile, the material exhibited excellent photoluminescent properties. Theoretical calculations further confirmed that the side-chain extension strategy suppressed radical dimerization through steric hindrance. The hexyl side chain reduced the HOMO-LUMO bandgap to facilitate electron transitions while increasing the molecular dihedral angle to minimize non-radiative recombination. The extension of the side chains effectively suppressed non-radiative transition pathways, ultimately enhanced both the photochromic and electrochromic performance.
通过偶联扩展和抑制二聚体增强咔唑-暴力分子的电致变色和光致发光
紫原具有可逆氧化还原特性和显著的显色性,是电致变色的核心材料。然而,它们的低共轭性降低了电荷转移效率,破坏了氧化还原可逆性,并导致荧光猝灭,从而限制了它们的应用。本研究通过提出“芳香桥接-柔性侧链”的协同设计策略来解决这一挑战。将富电子咔唑基团作为刚性桥接结构加入紫蛋白骨架中,扩展了分子偶联网络;而调整两侧烷基侧链的长度则抑制分子聚集。根据这一策略,我们设计并合成了以羟丙基(HICDP)和羟己基(HICDH)取代的咔唑-紫素衍生物。系统的研究揭示了咔唑单位和侧链长度如何调节它们的电致变色和光致发光性质。实验表明,咔唑桥接单元扩展了共轭体系,使HICDH在700 nm处的光学对比度达到ΔT = 77.4%。即使在100次循环后,HICDH仍保留了大约70%的光学对比度。侧链长度的增加增加了咔唑-紫素衍生物的自由基之间的距离,抑制了二聚体的形成,增强了稳定性。同时,该材料表现出优异的光致发光性能。理论计算进一步证实了侧链延伸策略通过位阻抑制自由基二聚化。己基侧链减小了HOMO-LUMO带隙以促进电子跃迁,同时增加了分子的二面角以减少非辐射重组。侧链的延伸有效地抑制了非辐射转变途径,最终提高了光致变色和电致变色性能。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Dyes and Pigments
工程技术-材料科学:纺织
CiteScore
8.20
自引率
13.30%
发文量
933
审稿时长
33 days
期刊介绍:
Dyes and Pigments covers the scientific and technical aspects of the chemistry and physics of dyes, pigments and their intermediates. Emphasis is placed on the properties of the colouring matters themselves rather than on their applications or the system in which they may be applied.
Thus the journal accepts research and review papers on the synthesis of dyes, pigments and intermediates, their physical or chemical properties, e.g. spectroscopic, surface, solution or solid state characteristics, the physical aspects of their preparation, e.g. precipitation, nucleation and growth, crystal formation, liquid crystalline characteristics, their photochemical, ecological or biological properties and the relationship between colour and chemical constitution. However, papers are considered which deal with the more fundamental aspects of colourant application and of the interactions of colourants with substrates or media.
The journal will interest a wide variety of workers in a range of disciplines whose work involves dyes, pigments and their intermediates, and provides a platform for investigators with common interests but diverse fields of activity such as cosmetics, reprographics, dye and pigment synthesis, medical research, polymers, etc.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


