An efficient amorphous ternary Ni-B-Ce catalyst for enhancing electrocatalytic oxidation performance of BH4−
IF 5.4
3区 化学
Q1 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
Abstract
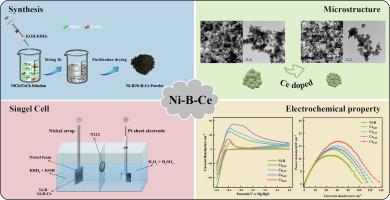
The development and utilization of anode catalysts are key to the advancement of direct borohydride fuel cells (DBFC). In this paper, Ni![]() B and Ni-B-Ce compounds were synthesized by the chemical reduction method. The electrocatalytic oxidation properties of Ni-B-Ce compound toward BH4− were systematically investigated. The microstructures of Ni
B and Ni-B-Ce compounds were synthesized by the chemical reduction method. The electrocatalytic oxidation properties of Ni-B-Ce compound toward BH4− were systematically investigated. The microstructures of Ni![]() B and Ni-B-Ce were characterized by inductively coupled plasma-optical emission spectrometer (ICP-OES), X-ray diffraction (XRD), scanning electron microscope (SEM), transmission electron microscope (TEM), and X-ray photoelectron spectroscopy (XPS). The electrochemical oxidation of BH4− was evaluated on Ni-B/NF and Ni-B-Ce/NF electrodes via cyclic voltammetry (CV), electrochemical impedance spectroscopy (EIS), chronoamperometry (CA), chronopotentiometry (CP), and electrochemical active surface area (ECSA) measurements. The results showed that the catalytic activity and stability of Ni-B-Ce were better than those of Ni
B and Ni-B-Ce were characterized by inductively coupled plasma-optical emission spectrometer (ICP-OES), X-ray diffraction (XRD), scanning electron microscope (SEM), transmission electron microscope (TEM), and X-ray photoelectron spectroscopy (XPS). The electrochemical oxidation of BH4− was evaluated on Ni-B/NF and Ni-B-Ce/NF electrodes via cyclic voltammetry (CV), electrochemical impedance spectroscopy (EIS), chronoamperometry (CA), chronopotentiometry (CP), and electrochemical active surface area (ECSA) measurements. The results showed that the catalytic activity and stability of Ni-B-Ce were better than those of Ni![]() B compound, and the Ce content affected the catalytic performance of Ni-B-Ce compound. The Ce0.25 catalyst was more conducive to the BOR reaction. To conclude, Ni
B compound, and the Ce content affected the catalytic performance of Ni-B-Ce compound. The Ce0.25 catalyst was more conducive to the BOR reaction. To conclude, Ni![]() B and Ni-B-Ce can be used as potential DBFC anode catalyst materials, and introducing rare earth element Ce improves the catalytic performance of Ni
B and Ni-B-Ce can be used as potential DBFC anode catalyst materials, and introducing rare earth element Ce improves the catalytic performance of Ni![]() B alloy.
B alloy.
一种提高BH4−电催化氧化性能的高效非晶态三元Ni-B-Ce催化剂
阳极催化剂的开发与利用是直接硼氢化物燃料电池(DBFC)发展的关键。本文采用化学还原法合成了NiB和Ni-B-Ce化合物。系统地研究了Ni-B-Ce化合物对BH4−的电催化氧化性能。采用电感耦合等离子体发射光谱仪(ICP-OES)、x射线衍射仪(XRD)、扫描电镜(SEM)、透射电镜(TEM)和x射线光电子能谱仪(XPS)对NiB和Ni-B-Ce的微观结构进行了表征。通过循环伏安法(CV)、电化学阻抗谱法(EIS)、计时电流法(CA)、计时电位法(CP)和电化学活性表面积(ECSA)测量,在Ni-B/NF和Ni-B- ce /NF电极上评价BH4−的电化学氧化。结果表明,Ni-B-Ce化合物的催化活性和稳定性优于NiB化合物,且Ce含量影响Ni-B-Ce化合物的催化性能。Ce0.25催化剂更有利于BOR反应。综上所述,NiB和Ni-B-Ce可以作为DBFC阳极催化剂材料,稀土元素Ce的引入提高了NiB合金的催化性能。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Inorganic Chemistry Communications
化学-无机化学与核化学
CiteScore
5.50
自引率
7.90%
发文量
1013
审稿时长
53 days
期刊介绍:
Launched in January 1998, Inorganic Chemistry Communications is an international journal dedicated to the rapid publication of short communications in the major areas of inorganic, organometallic and supramolecular chemistry. Topics include synthetic and reaction chemistry, kinetics and mechanisms of reactions, bioinorganic chemistry, photochemistry and the use of metal and organometallic compounds in stoichiometric and catalytic synthesis or organic compounds.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


