Synthesis of MOF-derived Ru/N-doped porous carbon for electrocatalytic hydrogen evolution reaction
IF 5.1
3区 材料科学
Q2 MATERIALS SCIENCE, COATINGS & FILMS
引用次数: 0
Abstract
The alkaline hydrogen evolution reaction (HER) presents a critical challenge in water electrolysis due to its inherently sluggish kinetics. To address this limitation, we developed a cage-confinement strategy (CCS) to precisely anchor ultrafine ruthenium (Ru) nanoclusters on metal-organic framework (MOF)-derived nitrogen-doped porous carbon (NPC), enabling simultaneous control over electronic structure and particle size distribution. The optimized Ru@NPC catalyst demonstrates exceptional HER performance, achieving a remarkably low overpotential of 81.6 mV at 10 mA cm−2 - a 5.3-fold improvement over the bare NPC support (436 mV). The material's superior reaction kinetics are further evidenced by its low Tafel slope of 51 mV dec−1, approaching the theoretical limit for Volmer-Heyrovsky mechanisms. Combined with excellent durability, these results establish Ru@NPC as a promising noble metal-efficient electrocatalyst for sustainable hydrogen production, demonstrating the effectiveness of our CCS approach in designing high-performance catalytic architectures.
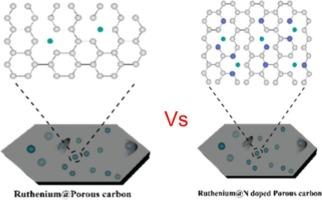
电催化析氢反应用mof衍生Ru/ n掺杂多孔碳的合成
碱性析氢反应(HER)由于其固有的缓慢动力学而成为水电解中的一个关键挑战。为了解决这一限制,我们开发了一种笼状约束策略(CCS)来精确地将超细钌(Ru)纳米团簇锚定在金属有机框架(MOF)衍生的氮掺杂多孔碳(NPC)上,从而同时控制电子结构和粒径分布。优化后的Ru@NPC催化剂表现出优异的HER性能,在10 mA cm - 2下实现了非常低的过电位81.6 mV,比裸NPC支持(436 mV)提高了5.3倍。该材料优越的反应动力学进一步证明了它的低Tafel斜率为51 mV dec−1,接近Volmer-Heyrovsky机制的理论极限。结合优异的耐久性,这些结果确立了Ru@NPC作为一种有前途的贵金属高效电催化剂,用于可持续制氢,证明了我们的CCS方法在设计高性能催化结构方面的有效性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Diamond and Related Materials
工程技术-材料科学:综合
CiteScore
6.00
自引率
14.60%
发文量
702
审稿时长
2.1 months
期刊介绍:
DRM is a leading international journal that publishes new fundamental and applied research on all forms of diamond, the integration of diamond with other advanced materials and development of technologies exploiting diamond. The synthesis, characterization and processing of single crystal diamond, polycrystalline films, nanodiamond powders and heterostructures with other advanced materials are encouraged topics for technical and review articles. In addition to diamond, the journal publishes manuscripts on the synthesis, characterization and application of other related materials including diamond-like carbons, carbon nanotubes, graphene, and boron and carbon nitrides. Articles are sought on the chemical functionalization of diamond and related materials as well as their use in electrochemistry, energy storage and conversion, chemical and biological sensing, imaging, thermal management, photonic and quantum applications, electron emission and electronic devices.
The International Conference on Diamond and Carbon Materials has evolved into the largest and most well attended forum in the field of diamond, providing a forum to showcase the latest results in the science and technology of diamond and other carbon materials such as carbon nanotubes, graphene, and diamond-like carbon. Run annually in association with Diamond and Related Materials the conference provides junior and established researchers the opportunity to exchange the latest results ranging from fundamental physical and chemical concepts to applied research focusing on the next generation carbon-based devices.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


