Comparative study of in vitro colonic fermentation dynamics: Human gut microbiota response to resistant starch derived from germinated and extruded pea seeds
IF 11
1区 农林科学
Q1 CHEMISTRY, APPLIED
引用次数: 0
Abstract
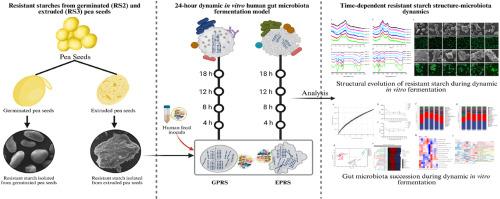
This study explores the temporal interaction between the multi-scale structural evolution of pea-derived resistant starches (RS) and gut microbiota dynamics using a dynamic in vitro colonic model. RS from germinated (GPRS, RS2) and extruded pea seeds (EPRS, RS3) were isolated and their molecular weight, crystallinity, and morphological transformations were characterized during 24-h fermentation. EPRS featured a porous, low-crystallinity matrix that enabled rapid microbial degradation, leading to an early enrichment of Bacteroidetes through starch utilization systems and propionate-dominant short-chain fatty acids (SCFAs) production. In contrast, GPRS preserved its structural integrity with higher molecular order, promoting gradual colonization by Firmicutes via ABC transporter-mediated oligosaccharide uptake and sustained butyrate production. Interestingly, EPRS exhibited higher total SCFAs production than GPRS during the 8–24 h fermentation period. Enzymatic profiling revealed substrate-dependent strategies: EPRS upregulated amylolytic enzymes (K01187) during initial Bacteroidetes dominance, whereas GPRS activated ABC transporters (K10111-K10112) for oligosaccharide internalization by Firmicutes. These results demonstrate that the structural organization of RS in pea seeds fundamentally governs microbiota-SCFAs interactions during colonic fermentation, with extrusion processing enhancing overall fermentability and germination maintaining the butyrate-producing activity. This mechanistic insight could provide a conceptual framework for the rational design of legume-derived prebiotics with tailored physiological benefits.
体外结肠发酵动力学的比较研究:人肠道菌群对发芽和挤压豌豆种子抗性淀粉的反应
本研究利用动态体外结肠模型探讨了豌豆源抗性淀粉(RS)的多尺度结构进化与肠道微生物群动力学之间的时间相互作用。从发芽豌豆种子(GPRS、RS2)和挤压豌豆种子(EPRS、RS3)中分离出RS,并对其分子量、结晶度和形态变化进行了表征。EPRS具有多孔、低结晶度的基质,能够快速进行微生物降解,通过淀粉利用系统和丙酸优势短链脂肪酸(SCFAs)的产生,导致拟杆菌门的早期富集。相比之下,GPRS以更高的分子顺序保持其结构完整性,促进厚壁菌门通过ABC转运蛋白介导的低聚糖摄取和持续的丁酸盐生产逐渐定植。有趣的是,在8-24 h发酵期间,EPRS的总SCFAs产量高于GPRS。酶分析揭示了底物依赖的策略:在初始拟杆菌门优势时,EPRS上调了淀粉水解酶(K01187),而GPRS激活了ABC转运蛋白(K10111-K10112),用于厚壁菌门的低聚糖内化。这些结果表明,豌豆种子中RS的结构组织从根本上控制了结肠发酵过程中微生物与scfas的相互作用,挤压处理提高了豌豆种子的整体发酵能力,而萌发过程保持了丁酸盐的产生活性。这种机制的洞察力可以为合理设计豆类衍生的益生元提供一个概念框架,这些益生元具有量身定制的生理益处。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Food Hydrocolloids
工程技术-食品科技
CiteScore
19.90
自引率
14.00%
发文量
871
审稿时长
37 days
期刊介绍:
Food Hydrocolloids publishes original and innovative research focused on the characterization, functional properties, and applications of hydrocolloid materials used in food products. These hydrocolloids, defined as polysaccharides and proteins of commercial importance, are added to control aspects such as texture, stability, rheology, and sensory properties. The research's primary emphasis should be on the hydrocolloids themselves, with thorough descriptions of their source, nature, and physicochemical characteristics. Manuscripts are expected to clearly outline specific aims and objectives, include a fundamental discussion of research findings at the molecular level, and address the significance of the results. Studies on hydrocolloids in complex formulations should concentrate on their overall properties and mechanisms of action, while simple formulation development studies may not be considered for publication.
The main areas of interest are:
-Chemical and physicochemical characterisation
Thermal properties including glass transitions and conformational changes-
Rheological properties including viscosity, viscoelastic properties and gelation behaviour-
The influence on organoleptic properties-
Interfacial properties including stabilisation of dispersions, emulsions and foams-
Film forming properties with application to edible films and active packaging-
Encapsulation and controlled release of active compounds-
The influence on health including their role as dietary fibre-
Manipulation of hydrocolloid structure and functionality through chemical, biochemical and physical processes-
New hydrocolloids and hydrocolloid sources of commercial potential.
The Journal also publishes Review articles that provide an overview of the latest developments in topics of specific interest to researchers in this field of activity.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


