Dynamics of disordered mackinawite (FeSm) at low temperatures and its geochemical implications
IF 8.9
1区 地球科学
Q1 GEOSCIENCES, MULTIDISCIPLINARY
引用次数: 0
Abstract
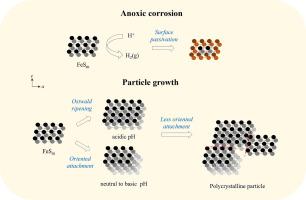
Disordered mackinawite (FeSm), an initial iron sulfide forming under ambient, anoxic conditions, plays a central role in sedimentary iron and sulfur cycling and may have contributed to early biochemical processes relevant to the origin of life. However, its structural variability complicates the assessments of its geochemical behavior and environmental impacts. Here, we demonstrate that FeSm undergoes anoxic corrosion at 25 °C, generating H2 even in the absence of traditional oxidants such as hydrogen sulfide (H2S) or elemental sulfur (S0). This abiotic H2 production provides a potential reductant for early Earth carbon fixation and may support modern oligotrophic ecosystems by influencing carbon cycling. The pH-dependent H2 production kinetics suggests that protons (H+) likely act as the primary oxidant in FeSm corrosion. The formation of Fe(III)-rich surface layers during this process passivates further corrosion and modulates surface reactivity—potentially facilitating the oxidation of H2S to S0 and intermediate species, thus driving FeSm transformation into greigite (Fe3S4) and pyrite (FeS2). Particle growth mechanisms vary with pH: Ostwald ripening dominates under acidic conditions, while oriented attachment is favored at neutral to alkaline pH. Instead, with prolonged aging, FeSm becomes stabilized through less-oriented attachment, producing polycrystalline particles. Both surface passivation and particle growth contribute to the resilience and dynamic behavior of FeSm under diverse geochemical conditions, reinforcing its role in sustaining iron and sulfur biogeochemical cycles. This study offers mechanistic insights into the structural evolution of FeSm, with implications for both early Earth environments and modern sedimentary systems.
低温下无序麦金石(FeSm)的动力学及其地球化学意义
无序硫化铁(FeSm)是一种在环境缺氧条件下形成的初始硫化铁,在沉积铁和硫循环中起着核心作用,可能对与生命起源相关的早期生化过程做出了贡献。然而,其结构变异性使其地球化学行为和环境影响的评估复杂化。在这里,我们证明了FeSm在25°C下经历缺氧腐蚀,即使在没有硫化氢(H2S)或单质硫(S0)等传统氧化剂的情况下也会产生H2。这种非生物制氢为早期地球碳固定提供了潜在的还原剂,并可能通过影响碳循环来支持现代少营养生态系统。ph依赖的H2生成动力学表明,质子(H+)可能是FeSm腐蚀中的主要氧化剂。在这一过程中,富Fe(III)表层的形成进一步钝化了腐蚀,并调节了表面反应性——可能促进H2S氧化为S0和中间物质,从而推动FeSm转化为灰长岩(Fe3S4)和黄铁矿(FeS2)。颗粒生长机制随pH值的变化而变化:酸性条件下奥斯特瓦尔德成熟占优势,而中性至碱性条件下有利于取向附着。相反,随着老化时间的延长,FeSm通过较少的取向附着而变得稳定,产生多晶颗粒。表面钝化和颗粒生长都有助于FeSm在不同地球化学条件下的弹性和动态行为,增强其在维持铁和硫生物地球化学循环中的作用。这项研究为FeSm的构造演化提供了机制上的见解,对早期地球环境和现代沉积体系都有启示。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Geoscience frontiers
Earth and Planetary Sciences-General Earth and Planetary Sciences
CiteScore
17.80
自引率
3.40%
发文量
147
审稿时长
35 days
期刊介绍:
Geoscience Frontiers (GSF) is the Journal of China University of Geosciences (Beijing) and Peking University. It publishes peer-reviewed research articles and reviews in interdisciplinary fields of Earth and Planetary Sciences. GSF covers various research areas including petrology and geochemistry, lithospheric architecture and mantle dynamics, global tectonics, economic geology and fuel exploration, geophysics, stratigraphy and paleontology, environmental and engineering geology, astrogeology, and the nexus of resources-energy-emissions-climate under Sustainable Development Goals. The journal aims to bridge innovative, provocative, and challenging concepts and models in these fields, providing insights on correlations and evolution.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


