1-(3,4-Diaminophenyl)-1H-pyrrole-2-carboxylic acids as potent apo-IDO1 inhibitors exhibiting efficacy in syngeneic tumor mouse models
IF 5.9
2区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
Abstract
Indolamine 2,3-dioxygenase 1 (IDO1)-mediated kynurenine pathway has been identified as an important immune escape mechanism in cancer, and pharmacological inhibition of IDO1 is regarded as a potential strategy for cancer treatment. Herein, we describe the identification of novel 1H-pyrrole-2-carboxylic acid-based IDO1 inhibitors targeting its apo form with picomolar potency in the HeLa cell-based assay. Among them, compound 45 showed the strongest inhibitory activity against IDO1 with an IC50 value of 10 pM. Notably, oral administration of 45 at 10 mg/kg significantly suppressed tumor growth by activating antitumor immunity without significant toxicity in a CT26 syngeneic mouse model. Furthermore, the tumor burden could similarly be decreased in an LLC syngeneic mouse model treated with 45, indicating the potential of 45 for the treatment of distinct tumor types. Collectively, these data suggest that compound 45 may be used as a promising lead compound for further investigation.
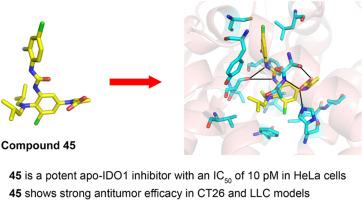
1-(3,4-二氨基苯基)- 1h -吡咯-2-羧酸是有效的载脂蛋白ido1抑制剂,在同基因肿瘤小鼠模型中显示有效
吲哚胺2,3-双加氧酶1 (IDO1)介导的犬尿氨酸途径已被确定为癌症中重要的免疫逃逸机制,药物抑制IDO1被认为是治疗癌症的潜在策略。在这里,我们描述了在HeLa细胞为基础的实验中,鉴定了一种新的以1h -吡咯-2-羧酸为基础的IDO1抑制剂,该抑制剂靶向其载脂蛋白形式,具有皮摩尔效力。其中化合物45对IDO1的抑制活性最强,IC50值为10 pM。值得注意的是,在CT26同基因小鼠模型中,口服45 (10 mg/kg)通过激活抗肿瘤免疫显著抑制肿瘤生长,且无明显毒性。此外,在45处理的LLC同基因小鼠模型中,肿瘤负荷同样可以减少,这表明45治疗不同类型肿瘤的潜力。综上所述,这些数据表明化合物45可能作为一种有前途的先导化合物进行进一步的研究。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
11.70
自引率
9.00%
发文量
863
审稿时长
29 days
期刊介绍:
The European Journal of Medicinal Chemistry is a global journal that publishes studies on all aspects of medicinal chemistry. It provides a medium for publication of original papers and also welcomes critical review papers.
A typical paper would report on the organic synthesis, characterization and pharmacological evaluation of compounds. Other topics of interest are drug design, QSAR, molecular modeling, drug-receptor interactions, molecular aspects of drug metabolism, prodrug synthesis and drug targeting. The journal expects manuscripts to present the rational for a study, provide insight into the design of compounds or understanding of mechanism, or clarify the targets.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


