Nano-Self-Assembled Particles Loaded with Aldehyde Dehydrogenase 2 Agonist Inhibit Ischemia-Reperfusion Injury of Transplanted Kidneys
IF 16
1区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Kidney transplantation serves as an ideal treatment for patients with end-stage renal disease. Due to the organ shortage, there has been an uptick in the use of circulatory death donors (DCD), yet DCD kidneys endure severe ischemia-reperfusion injury (IRI), limiting transplantation efficacy. Therefore, we developed a strategy combining hypothermic machine perfusion (HMP) with aldehyde dehydrogenase 2 (ALDH2) agonist Alda-1, using quaternized chitosan/5β-cholanic acid/Alda-1 nanoparticles (A-NCH) for ex vivo DCD kidney repair. Methodologically, A-NCH was synthesized by conjugating O-hydroxypropyl trimethylammonium chloride chitosan (O-HTCC) with 5β-cholanic acid via EDC/NHS-mediated coupling, forming self-assembled micelles with a mean diameter of 132.4 ± 0.3 nm and ζ-potential of 45.0 ± 1.0 mV. It exhibited a high drug loading ratio (41.9%), with 80% of Alda-1 released at 4 °C within 3.5 h following the Weibull model, enabling sustained drug delivery during HMP. A-NCH demonstrated outstanding compatibility with cells and blood, as well as effective antibacterial properties combat Escherichia coli and Staphylococcus aureus. In vitro, A-NCH reduced H2O2- and oxygen-glucose deprivation- and -reoxygenation-induced oxidative stress and apoptosis in HK-2 and HUVECs. In vivo, using a rat DCD kidney transplantation model, A-NCH-administered HMP accelerated kidney graft function recovery and alleviated renal tubular injury. Mechanistically, A-NCH activated ALDH2, inhibited the P38 MAPK pathway, promoted nuclear translocation of TEAD4/YAP1, and suppressed the transition of proximal tubule cells to an injured phenotype. In this study, the solubility and drug loading of Alda-1 were improved by 5β-cholanic acid modification of O-HTCC, which proved the synergistic efficacy with HMP in DCD kidney repair and provided a translatable strategy to expand the donor pool.
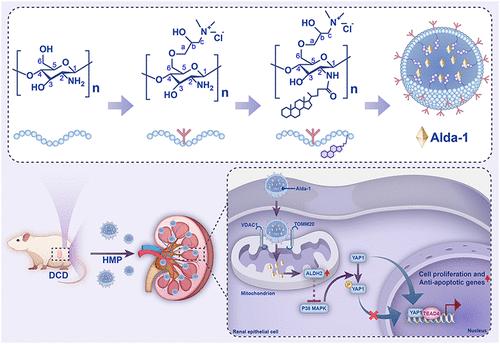
负载醛脱氢酶2激动剂的纳米自组装颗粒抑制移植肾缺血再灌注损伤
肾移植是终末期肾病患者的理想治疗方法。由于器官短缺,使用循环死亡供体(DCD)的情况有所增加,但DCD肾脏存在严重的缺血再灌注损伤(IRI),限制了移植的效果。因此,我们开发了一种结合低温机器灌注(HMP)和醛脱氢酶2 (ALDH2)激动剂Alda-1的策略,使用季铵化壳聚糖/5β-胆酸/Alda-1纳米颗粒(a- nch)进行体外DCD肾脏修复。方法上,通过EDC/ nhs介导偶联,将o -羟丙基三甲基氯化铵壳聚糖(O-HTCC)与5β-胆酸偶联合成a - nch,形成平均直径为132.4±0.3 nm, ζ电位为45.0±1.0 mV的自组装胶束。它具有很高的载药率(41.9%),在Weibull模型后的3.5 h内,80%的Alda-1在4°C下释放,从而在HMP期间持续给药。A-NCH表现出与细胞和血液的出色相容性,以及对抗大肠杆菌和金黄色葡萄球菌的有效抗菌性能。在体外,A-NCH可降低HK-2和HUVECs中H2O2-和氧葡萄糖剥夺-和-再氧诱导的氧化应激和凋亡。在体内,采用大鼠DCD肾移植模型,a - nch给药的HMP加速了肾移植功能的恢复,减轻了肾小管损伤。在机制上,A-NCH激活ALDH2,抑制P38 MAPK通路,促进TEAD4/YAP1的核易位,抑制近端小管细胞向损伤表型的转变。本研究通过对O-HTCC进行5β-胆酸修饰,提高了Alda-1的溶解度和载药量,证明了与HMP在DCD肾修复中的协同作用,为扩大供体池提供了可翻译的策略。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Nano
工程技术-材料科学:综合
CiteScore
26.00
自引率
4.10%
发文量
1627
审稿时长
1.7 months
期刊介绍:
ACS Nano, published monthly, serves as an international forum for comprehensive articles on nanoscience and nanotechnology research at the intersections of chemistry, biology, materials science, physics, and engineering. The journal fosters communication among scientists in these communities, facilitating collaboration, new research opportunities, and advancements through discoveries. ACS Nano covers synthesis, assembly, characterization, theory, and simulation of nanostructures, nanobiotechnology, nanofabrication, methods and tools for nanoscience and nanotechnology, and self- and directed-assembly. Alongside original research articles, it offers thorough reviews, perspectives on cutting-edge research, and discussions envisioning the future of nanoscience and nanotechnology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


