Electrochemical and Mechanical Evolution of Sulfide-Based Solid Electrolytes: Insights from Operando XPS and Cell Pressure Measurements
IF 12.1
2区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
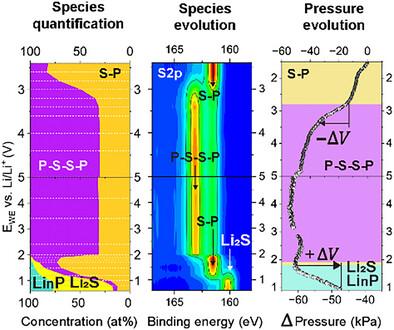
Understanding the electrochemical and mechanical behavior of solid electrolytes beyond their electrochemical stability window is crucial for enabling high energy density all-solid-state batteries. Accordingly, this work systematically studies a model working electrode of Li3PS4, ball milled with vapor grown carbon fiber (VGCF). Operando X-ray photoelectron spectroscopy can identify and quantify the potential-dependent redox byproducts, their reversibility, and electrical properties, while operando cell pressure measurements correlate these with volume changes and mechanical instability. The study examines voltages up to 5.0 V and down to −0.05 V versus Li/Li+, mimicking cathode and anode cycling. It demonstrates that within the 2.4–5.0 V region, Li3PS4 oxidation byproducts are primarily polysulfides composed of bridging sulfurs (P-S-S-P) between PS43- units, free of elemental sulfur (S0), and electrically conductive. The Li3PS4 oxidation process occurs at 2.8 V during first charge and ends at 3.4 V, with volume shrinkage at the VGCF interface. During reduction (2.4 to −0.05 V), polysulfides convert reversibly to Li3PS4 between 1.9 and 1.7 V, then to Li2S and LinP (0 ≤ n ≤ 3) between 1.9 and 0.6 V, causing volume expansion and the transition to an electrically insulating interphase. Below 0.6 V, Li2O formation dominates without further evolution of Li2S or LinP.
硫化物基固体电解质的电化学和力学演化:来自Operando XPS和电池压力测量的见解
了解固体电解质在其电化学稳定窗口之外的电化学和机械行为对于实现高能量密度的全固态电池至关重要。因此,本工作系统地研究了气相生长碳纤维(VGCF)球磨Li3PS4模型工作电极。Operando x射线光电子能谱可以识别和量化电位依赖的氧化还原副产物、它们的可逆性和电学性质,而Operando细胞压力测量将这些与体积变化和机械不稳定性联系起来。该研究测试了高达5.0 V和低至- 0.05 V的电压与Li/Li+,模拟阴极和阳极循环。结果表明,在2.4 ~ 5.0 V范围内,Li3PS4氧化副产物主要是由PS43-单元之间的桥接硫(P-S-S-P)组成的多硫化物,不含单质硫(S0),具有导电性。Li3PS4的氧化过程在首次充电时发生在2.8 V,在3.4 V时结束,在VGCF界面处体积收缩。在还原(2.4 ~−0.05 V)过程中,多硫化物在1.9 ~ 1.7 V之间可逆地转化为Li3PS4,然后在1.9 ~ 0.6 V之间可逆地转化为Li2S和LinP(0≤n≤3),导致体积膨胀并过渡到电绝缘界面。在0.6 V以下,Li2O形成占主导地位,Li2S和LinP不再进一步演化。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Small
工程技术-材料科学:综合
CiteScore
17.70
自引率
3.80%
发文量
1830
审稿时长
2.1 months
期刊介绍:
Small serves as an exceptional platform for both experimental and theoretical studies in fundamental and applied interdisciplinary research at the nano- and microscale. The journal offers a compelling mix of peer-reviewed Research Articles, Reviews, Perspectives, and Comments.
With a remarkable 2022 Journal Impact Factor of 13.3 (Journal Citation Reports from Clarivate Analytics, 2023), Small remains among the top multidisciplinary journals, covering a wide range of topics at the interface of materials science, chemistry, physics, engineering, medicine, and biology.
Small's readership includes biochemists, biologists, biomedical scientists, chemists, engineers, information technologists, materials scientists, physicists, and theoreticians alike.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


