High-Performance Mn-Based Hybrid Aqueous Batteries Enabled by Interlayer-Expanded Magnesium Vanadium Bronze Cathode
IF 12.1
2区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Manganese-based ion batteries exhibit a relatively low redox potential (−1.19 V vs SHE) along with high theoretical capacity and excellent capacity retention characteristics, making them promising for next-generation batteries. In this study, a high-performance manganese hybrid aqueous battery with an expanded interlayer spacing is designed and fabricated using MgV6O16·7H2O (MgVO) as the cathode material and manganese metal as the anode. The fabricated Mn metal battery achieves an initial discharge capacity of 195.3 mAh g−1 (with Mn metal anode) at a current density of 0.1 A g−1 with 1.19 V and exhibits excellent capacity retention of even after 200 cycles at a current density of 0.4 A g−1. Furthermore, detailed structural behavior and operational mechanisms of ions within the MgVO electrode are elucidated through comprehensive analyses of the crystal structure, ion diffusion pathways, and activation energy calculations. Compared to zinc-based aqueous batteries (−0.76 V vs SHE), the manganese-based system demonstrates a ≈0.43 V higher theoretical operating voltage, which is consistent with the experimental observations. This study not only confirms the potential of MgVO as a promising cathode material for next-generation manganese aqueous batteries but also provides valuable insights for the performance enhancement and design optimization of manganese-based aqueous battery systems.
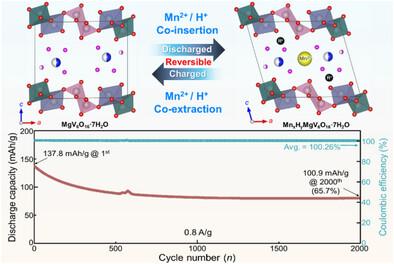
层间膨胀镁钒青铜阴极实现高性能锰基混合水电池
锰基离子电池具有相对较低的氧化还原电位(- 1.19 V vs SHE)、较高的理论容量和优异的容量保持特性,有望成为下一代电池。本研究以MgV6O16·7H2O (MgVO)为正极材料,金属锰为阳极,设计并制备了一种扩展层间距的高性能锰杂化水电池。制备的锰金属电池在1.19 V电流密度为0.1 a g−1时,具有195.3 mAh g−1的初始放电容量(Mn金属阳极),在0.4 a g−1电流密度下,即使在200次循环后也能保持良好的容量。此外,通过晶体结构、离子扩散途径和活化能计算的综合分析,阐明了MgVO电极内离子的详细结构行为和运行机制。与锌基水电池(−0.76 V vs SHE)相比,锰基系统的理论工作电压高约0.43 V,与实验观察结果一致。该研究不仅证实了MgVO作为下一代锰基水电池正极材料的潜力,而且为锰基水电池系统的性能增强和设计优化提供了有价值的见解。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Small
工程技术-材料科学:综合
CiteScore
17.70
自引率
3.80%
发文量
1830
审稿时长
2.1 months
期刊介绍:
Small serves as an exceptional platform for both experimental and theoretical studies in fundamental and applied interdisciplinary research at the nano- and microscale. The journal offers a compelling mix of peer-reviewed Research Articles, Reviews, Perspectives, and Comments.
With a remarkable 2022 Journal Impact Factor of 13.3 (Journal Citation Reports from Clarivate Analytics, 2023), Small remains among the top multidisciplinary journals, covering a wide range of topics at the interface of materials science, chemistry, physics, engineering, medicine, and biology.
Small's readership includes biochemists, biologists, biomedical scientists, chemists, engineers, information technologists, materials scientists, physicists, and theoreticians alike.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


