Chemodivergent Synthesis of 1,3-Benzoxazines, Indolin-3-ols, and Indoles from the Designer Amides: Applications to the Synthesis of Phenanthridine Natural Products
IF 3.6
2区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
A chemodivergent synthesis of 1,3-benzoxazines, indolin-3-ols, and indoles has been conceptualized from readily accessible 2-bromoacetylphenyl benzamides. The strategy emanates from the NaBH4-mediated reduction of the designer amides, triggering an intramolecular cyclization cascade and furnishing three distinct classes of heterocycles with high precision. A unified synthesis of phenanthridine natural products was also accomplished to work out the utility of this strategy. Broad substrate scope, operational flexibility, and successful implementation at the gram-scale level are notable highlights of this strategy.
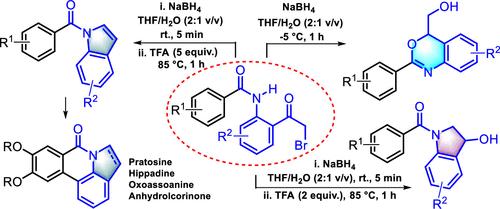
从设计体酰胺合成1,3-苯并恶嗪、吲哚-3-醇和吲哚的化学发散法:在菲咯啉天然产物合成中的应用
化学发散合成1,3-苯并恶嗪,吲哚-3-醇和吲哚的概念已经从容易获得的2-溴乙酰苯基苯并胺。该策略源于nabh4介导的设计酰胺的还原,触发分子内环化级联,并以高精度提供三种不同类型的杂环。并对菲咯啶天然产物进行了统一合成,以验证该策略的实用性。广泛的衬底范围、操作灵活性和克级的成功实施是该策略的显著亮点。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


