Stereoselective Synthesis of 2-Deoxy-α-O-glycosides Enabled by Cobalt-Catalyzed Hydrogen Atom Transfer and Radical-Polar Crossover
IF 13.1
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
We report a stereocontrolled glycosylation strategy for synthesizing 2-deoxy-α-O-glycosides via cobalt-catalyzed metal-hydride hydrogen atom transfer (MHAT) and radical-polar crossover (RPC) processes. This method achieves reagent-controlled stereoselectivity by modulating ligand environments, silane additives, and solvent effects, eliminating dependence on neighboring-group participation. Mechanistic studies reveal a sequence involving Co(III)–H-mediated hydrogen atom transfer, radical generation, and nucleophilic trapping of a Co(IV)–R intermediate to establish α-selectivity. Compared to conventional glycosylation protocols, this multitunable platform and chemoselectively compatible reaction conditions enable flexible 2-deoxy sugar assembly with extensive functional group tolerance, especially for substrates containing basic nitrogenous functional groups. The methodology provides a versatile and mild foundation for constructing structurally diverse glycoconjugates, including steroidal glycosides and nitrogen-containing acceptors such as ribonucleosides, deoxyribonucleosides, aniline, pyrrole, indole, and carbazole, demonstrating considerable potential for bioactive molecule development.
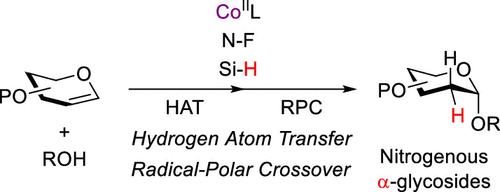
钴催化氢原子转移和自由基极性交叉使2-脱氧-α- o -糖苷的立体选择性合成成为可能
我们报道了一种立体控制糖基化策略,通过钴催化金属氢化物氢原子转移(MHAT)和自由基-极性交叉(RPC)过程合成2-脱氧-α- o -糖苷。该方法通过调节配体环境、硅烷添加剂和溶剂效应来实现试剂控制的立体选择性,消除了对邻基参与的依赖。机制研究揭示了Co(III) - h介导的氢原子转移、自由基生成和Co(IV) -R中间体的亲核捕获等一系列过程,以建立α-选择性。与传统的糖基化方案相比,这种多可调平台和化学选择性相容的反应条件使灵活的2-脱氧糖组装具有广泛的官能团耐受性,特别是对于含有碱性氮官能团的底物。该方法为构建结构多样的糖缀合物(包括甾体糖苷和含氮受体,如核糖核苷、脱氧核糖核苷、苯胺、吡咯、吲哚和咔唑)提供了通用和温和的基础,显示出相当大的生物活性分子开发潜力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Catalysis
CHEMISTRY, PHYSICAL-
CiteScore
20.80
自引率
6.20%
发文量
1253
审稿时长
1.5 months
期刊介绍:
ACS Catalysis is an esteemed journal that publishes original research in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. It offers broad coverage across diverse areas such as life sciences, organometallics and synthesis, photochemistry and electrochemistry, drug discovery and synthesis, materials science, environmental protection, polymer discovery and synthesis, and energy and fuels.
The scope of the journal is to showcase innovative work in various aspects of catalysis. This includes new reactions and novel synthetic approaches utilizing known catalysts, the discovery or modification of new catalysts, elucidation of catalytic mechanisms through cutting-edge investigations, practical enhancements of existing processes, as well as conceptual advances in the field. Contributions to ACS Catalysis can encompass both experimental and theoretical research focused on catalytic molecules, macromolecules, and materials that exhibit catalytic turnover.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


