High-Entropy Solvation Configurations toward Practical Fast-Charging and Safe Lithium-Ion Batteries
IF 18.2
1区 材料科学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
Severe performance decay is witnessed for fast-charging lithium-ion batteries due to tremendous kinetic barriers. Herein, high-entropy solvation configurations are achieved to diminish such kinetic limitations by inducing multiple salts into the electrolyte. Theoretical calculations combined with experimental characterizations confirm the enhanced entropy of the electrolyte, rendering the participation of several anionic groups and reducing the solvation strength between Li+ and solvent/anion species. Concurrently, inorganic-rich interphases are further identified via preferential oxidation/reduction of diverse multianions clusters, thus reducing the interphases impedance. The graphite/LiNi0.5Mn0.2Co0.3O2 full cell maintains 95.4% capacity retention over 650 cycles at 1 C and delivers a capacity of 82.3 mAh g–1 (45.2 mAh g–1 for routine batteries) at 5 C. Such kinetic performance ensures the safety without Li plating at fast charging, increasing the thermal failure onset temperature from 68.6 °C to 167.7 °C under deeply aging conditions. This electrolyte offers an opportunity to achieve safe and long-term fast-charging lithium-ion batteries.
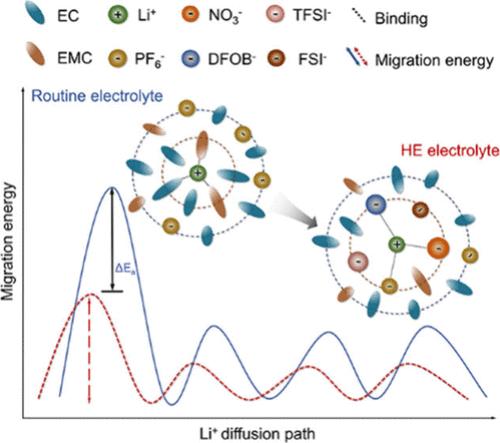
实用快速充电和安全锂离子电池的高熵溶剂化配置
由于巨大的动力学障碍,快速充电锂离子电池的性能衰减严重。在这里,高熵溶剂化结构通过诱导多种盐进入电解质来减少这种动力学限制。理论计算与实验表征相结合,证实了电解质熵的增强,导致多个阴离子基团的参与,降低了Li+与溶剂/阴离子之间的溶剂化强度。同时,通过不同多阴离子团簇的优先氧化/还原,进一步确定了富无机界面相,从而降低了界面阻抗。石墨/LiNi0.5Mn0.2Co0.3O2全电池在1℃下循环650次,保持95.4%的容量,在5℃下提供82.3 mAh g-1的容量(常规电池为45.2 mAh g-1),这样的动力学性能保证了快速充电时不镀锂的安全性,并将深度老化条件下的热失效起始温度从68.6℃提高到167.7℃。这种电解质为实现安全和长期快速充电的锂离子电池提供了机会。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Energy Letters
Energy-Renewable Energy, Sustainability and the Environment
CiteScore
31.20
自引率
5.00%
发文量
469
审稿时长
1 months
期刊介绍:
ACS Energy Letters is a monthly journal that publishes papers reporting new scientific advances in energy research. The journal focuses on topics that are of interest to scientists working in the fundamental and applied sciences. Rapid publication is a central criterion for acceptance, and the journal is known for its quick publication times, with an average of 4-6 weeks from submission to web publication in As Soon As Publishable format.
ACS Energy Letters is ranked as the number one journal in the Web of Science Electrochemistry category. It also ranks within the top 10 journals for Physical Chemistry, Energy & Fuels, and Nanoscience & Nanotechnology.
The journal offers several types of articles, including Letters, Energy Express, Perspectives, Reviews, Editorials, Viewpoints and Energy Focus. Additionally, authors have the option to submit videos that summarize or support the information presented in a Perspective or Review article, which can be highlighted on the journal's website. ACS Energy Letters is abstracted and indexed in Chemical Abstracts Service/SciFinder, EBSCO-summon, PubMed, Web of Science, Scopus and Portico.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


