Detection and proteomic identification of in vivo S-nitrosylated proteins in Vibrio cholerae: A novel evidence
IF 3.2
2区 生物学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
Conserved across the phylogeny, S-nitrosylation and S-denitrosylation of biological thiols is a reversible protein post-translational modification of cysteine thiol residues involving nitric oxide (NO) and NO•-derived metabolites. S-nitrosylation of proteins is observed to transduce signalling pathways with significant pathological and physiological relevance. Although endogenous S-nitrosylation is also an obligate non-classical cellular signalling pathway of NO• in single-celled organisms, but very little information is available in prokaryotes. Hitherto unknown, we present experimental evidence for the first time in vivo S-nitrosylation (without using any NO•/RNS donor) of proteins of the enteropathogenic, Gram-negative bacteria O1 El Tor strains of Vibrio cholerae N16961 and C6706. In the present study, PSNO was quantified by 2, 3-diaminonaphthalene (DAN) using a spectrofluorometer, which was further supported by confocal microscopy. Western blot and mass spectrometry-based proteomic analyses identified ten S-nitrosylated proteins via DMPO-nitrone adduct formation. V. cholerae contained high amounts of the in vivo pool of S-nitrosylated proteome in different respiratory conditions. Experimental evidence shows that physiological levels of glutathione (GSH) can efficiently S-denitrosylate Vibrio cholerae PSNO in a concentration-dependent manner, suggesting that the intracellular GSH tends to reset the redox state of these protein thiols. Our data suggests that V. cholerae possesses more amount of in vivo PSNO during semi-anaerobic respiration than aerobic respiration and irrespective of media and strain used; stationary phase cells are relatively more stable to GSH-catalyzed S-denitrosylation than their log-phase counterparts. Additionally, the in vivo PSNO accumulation was found to be elevated in the nitrate reductase deletion mutant (ΔnapA), indicating the role of napA in the nitroso-oxidative stress response mechanism of V. cholerae. This could aid in its remarkable adaptability and survivability in the hostile conditions of the human intestine, thereby paving the way for cholera, a highly contagious diarrheal disease.
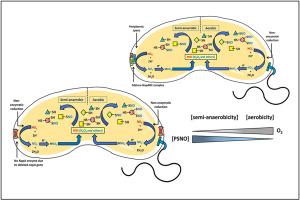
霍乱弧菌体内s -亚硝基化蛋白的检测和蛋白质组学鉴定:一个新的证据。
生物硫醇的s -亚硝基化和s -脱硝基化在整个系统发育中都是保守的,是一种涉及一氧化氮(NO)和一氧化氮衍生代谢产物的半胱氨酸硫醇残基的可逆蛋白质翻译后修饰。观察到蛋白质的s -亚硝基化转导信号通路具有显著的病理和生理相关性。虽然内源性s -亚硝基化在单细胞生物中也是NO•的专性非经典细胞信号通路,但在原核生物中可获得的信息很少。迄今为止,我们首次在体内(不使用任何NO•/RNS供体)证明了肠致病性革兰氏阴性菌o1el - Tor霍乱弧菌N16961和C6706的蛋白质的s -亚硝基化。在本研究中,PSNOs是用2,3 -二氨基萘(DAN)荧光光谱仪定量的,并通过共聚焦显微镜进一步支持。基于Western blot和质谱的蛋白质组学分析通过DMPO-nitrone加合物形成鉴定了10个s -亚硝基化蛋白。在不同呼吸条件下,霍乱弧菌体内含有大量的s -亚硝基化蛋白质组。实验证据表明,生理水平的谷胱甘肽(GSH)可以有效地以浓度依赖的方式对霍乱弧菌PSNOs进行s -脱硝,这表明细胞内的谷胱甘肽倾向于重置这些蛋白质硫醇的氧化还原状态。我们的数据表明,霍乱弧菌在半无氧呼吸过程中比在有氧呼吸过程中具有更多的体内PSNOs量,并且与使用的介质和菌株无关;相对于对数相细胞,固定相细胞对gsh催化的s -脱硝基化反应更稳定。此外,在硝酸还原酶缺失突变体中发现体内PSNOs积累增加(ΔnapA),表明napA在霍乱弧菌亚硝基氧化应激反应机制中的作用。这可能有助于它在人类肠道恶劣条件下的卓越适应性和生存能力,从而为霍乱这种高度传染性腹泻疾病铺平道路。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nitric oxide : biology and chemistry
生物-生化与分子生物学
CiteScore
7.50
自引率
7.70%
发文量
74
审稿时长
52 days
期刊介绍:
Nitric Oxide includes original research, methodology papers and reviews relating to nitric oxide and other gasotransmitters such as hydrogen sulfide and carbon monoxide. Special emphasis is placed on the biological chemistry, physiology, pharmacology, enzymology and pathological significance of these molecules in human health and disease. The journal also accepts manuscripts relating to plant and microbial studies involving these molecules.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


