Glycyrrhetinic acid orchestrates ERS-pyroptosis crosstalk to elicit immunogenic cell death in hepatocellular carcinoma
IF 8.3
1区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
Abstract
Background
Hepatocellular carcinoma (HCC) remains a magnitude global public health challenges since its aggressive biological behavior and persistently high morbidity and mortality rates. Glycyrrhetinic acid (GA), a bioactive triterpenoid extracted from Glycyrrhiza glabra, has been reported in certain contexts to suppress HCC cell migration and proliferation. In addition, the markedly immunosuppressive tumor microenvironment of HCC represents a major obstacle to achieving durable responses with immunotherapy.
Purpose
This study focused on elucidating the anti-hepatoma activity of GA, uncover the underlying molecular pathways, and assess its ability to trigger immunogenic cell death (ICD) as a potential therapeutic strategy for HCC.
Methods
Tumor-suppressive effects of GA were evaluated through histopathological analysis in multiple murine models, including cell line-derived xenografts (CDX), orthotopic liver implantation, and lung metastasis models. Transmission electron microscopy (TEM), intracellular calcium (Ca2+) assays, ROS staining, and immunofluorescence were used to investigate GA-induced endoplasmic reticulum stress (ERS) and pyroptosis in HCC cells. Proteomics and transcriptomics were applied to profile changes in protein and transcript expression. CETSA, DARTS, pull-down and molecular docking were employed to identify the direct molecular target of GA.
Results
The studies suggested that GA exhibited considerably similar anticancer efficacy (IRT = 46.62 %) compared with DOX (IRT = 48.87 %) in a CDX mouse model. Additionally, GA robustly activated ERS via PERK signaling and induced GSDME-mediated pyroptosis, increasing the cytoplasmic Ca2+ concentration, driving the translocation of calreticulin (83.32 %) to the tumor cell surface, resulting in the strong release of proinflammatory cytokines and immunogenic signals. This coordinated interaction enhanced DC maturation and T-cell dependent adaptive immune responses, amplifying antitumor immunity.
Conclusion
Our findings highlight a novel mechanism whereby GA exploits the ERS-pyroptosis axis to potentiate ICD and amplify antitumor immunity in HCC, providing mechanistic insight and potential therapeutic implications.
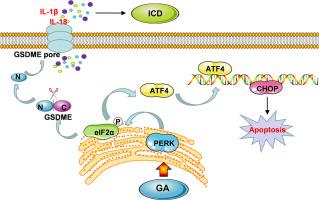
甘草次酸协调ers -焦亡串扰引起肝细胞癌免疫原性细胞死亡。
背景:肝细胞癌(HCC)由于其具有侵袭性的生物学行为和持续的高发病率和死亡率,仍然是一个重大的全球公共卫生挑战。甘草次酸(Glycyrrhetinic acid, GA)是一种从甘草中提取的具有生物活性的三萜,已被报道在某些情况下抑制肝癌细胞的迁移和增殖。此外,HCC明显的免疫抑制肿瘤微环境是实现免疫治疗持久反应的主要障碍。目的:本研究的重点是阐明GA的抗肝癌活性,揭示潜在的分子途径,并评估其触发免疫原性细胞死亡(ICD)的能力,作为HCC的潜在治疗策略。方法:采用多种小鼠模型,包括细胞系来源的异种移植(CDX)、原位肝移植和肺转移模型,通过组织病理学分析评估GA的肿瘤抑制作用。采用透射电镜(TEM)、细胞内钙(Ca2+)测定、ROS染色和免疫荧光法研究ga诱导的肝癌细胞内质网应激(ERS)和焦亡。蛋白质组学和转录组学应用于蛋白质和转录物表达的变化。采用CETSA、dart、pull-down、分子对接等方法鉴定GA的直接分子靶点。结果:研究表明,在CDX小鼠模型中,与DOX (IRT = 48.87%)相比,GA具有相当相似的抗癌功效(IRT = 46.62%)。此外,GA通过PERK信号强烈激活ERS,诱导gsdme介导的焦亡,增加细胞质Ca2+浓度,驱动钙网蛋白(83.32%)易位到肿瘤细胞表面,导致促炎细胞因子和免疫原性信号的强烈释放。这种协调的相互作用增强了DC成熟和t细胞依赖的适应性免疫反应,增强了抗肿瘤免疫。结论:我们的研究结果强调了一种新的机制,即GA利用ers -焦亡轴增强HCC的ICD和增强抗肿瘤免疫,提供了机制见解和潜在的治疗意义。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Phytomedicine
医学-药学
CiteScore
10.30
自引率
5.10%
发文量
670
审稿时长
91 days
期刊介绍:
Phytomedicine is a therapy-oriented journal that publishes innovative studies on the efficacy, safety, quality, and mechanisms of action of specified plant extracts, phytopharmaceuticals, and their isolated constituents. This includes clinical, pharmacological, pharmacokinetic, and toxicological studies of herbal medicinal products, preparations, and purified compounds with defined and consistent quality, ensuring reproducible pharmacological activity. Founded in 1994, Phytomedicine aims to focus and stimulate research in this field and establish internationally accepted scientific standards for pharmacological studies, proof of clinical efficacy, and safety of phytomedicines.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


