Angelica sinensis and Scutellaria baicalensis synergistically alleviates metabolic dysfunction-associated steatohepatitis via promoting adipose tissue-to-liver tissue communication
IF 8.3
1区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
Abstract
Background
The synergistic use of Danggui (Angelica sinensis, As) and Huangqin (Scutellaria baicalensis or Scutellaria baicalensis Georgi, SbG; As-SbG) exhibits a notable therapeutic effect on metabolic dysfunction-associated steatohepatitis (MASH). However, the underlying pharmacological mechanisms of their synergistic action have not yet been fully elucidated.
Purpose
This study aims to evaluate the therapeutic efficacy of the synergistic application of As-SbG on MASH induced by various dietary patterns, and to identify the specific potential active compounds, underlying pharmacological mechanisms, and key therapeutic targets responsible for its anti-MASH effects.
Methods
Two MASH animal models induced by methionine- and choline- deficient (MCD) diet and high-fat/high-cholesterol (HFHC) diet were constructed to compare the efficacy of As, SbG and As-SbG synergistic treatment on adipose tissue and liver tissue during MASH progression. Potential mechanistic pathways and key molecular targets were identified through proteomics analysis. The chemical components of As-SbG were characterized using ultrahigh performance liquid chromatography-high resolution mass spectrometry (UPLC-![]() HRMS). The interactions between the identified bioactive components and the key targets from proteomics were further validated using molecular docking methods, focusing on As-SbG’s therapeutic potential. Based on these bioinformatics findings, qRT-PCR, immunofluorescence staining, and Western blotting were employed to elucidate the regulatory mechanisms of As-SbG combination therapy on lipid metabolism, inflammation, and apoptosis-related pathways in both in vitro and in vivo MASH models.
HRMS). The interactions between the identified bioactive components and the key targets from proteomics were further validated using molecular docking methods, focusing on As-SbG’s therapeutic potential. Based on these bioinformatics findings, qRT-PCR, immunofluorescence staining, and Western blotting were employed to elucidate the regulatory mechanisms of As-SbG combination therapy on lipid metabolism, inflammation, and apoptosis-related pathways in both in vitro and in vivo MASH models.
Results
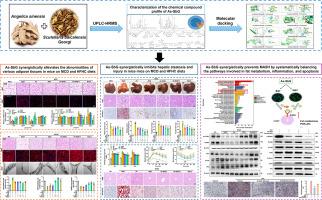
Compared with single-flavor administration of As and SbG, As-SbG synergistic administration significantly restored the balance among different phenotypes, functions, and sizes of various adipose tissues in MASH-induced mice under different dietary conditions, ameliorated hepatic steatosis and liver damage. Proteomic analysis revealed that the potential synergistic therapeutic effects of As-SbG on MASH were primarily associated with pathways involved in fat metabolism, anti-inflammatory responses, and anti-apoptotic mechanisms. Mechanism exploration revealed that the As-SbG synergistic therapy enhanced the expression of Nrg4 in adipose tissue, which specifically bound to ErbB receptors in liver tissue. This interaction activated the downstream PI3K/Akt, Ras/MAPK, and P53 signaling pathways, thereby promoting inter-tissue communication between adipose and liver tissues. Consequently, this regulatory effect improved hepatic lipid metabolism, suppressed inflammatory responses, and reduced hepatocellular damage, fibrosis, and apoptosis, ultimately ameliorating MASH. Furthermore, pharmacological inhibition of the key target ErbB4 in vitro abolished the protective effects of the As-SbG synergistic therapy against MASH. Additionally, a total of 31 chemical components of As-SbG were identified using UPLC![]() HRMS. Molecular docking analyses confirmed that Levistilide A, a compound derived from As, exhibits strong binding affinity with multiple key targets involved in various signaling pathways, including fat metabolism, anti-inflammatory responses, and anti-apoptotic mechanisms. Several bioactive compounds identified in SbG, such as Baicalein, Scutellarin, Oroxindin, and Neobaicalein, also demonstrated favorable interactions with these pathway-associated targets. These findings provide robust evidence supporting the hypothesis that the synergistic application of As-SbG exerts therapeutic effects on MASH through a multi-component, multi-pathway, and multi-target mechanism, highlighting a novel approach of traditional Chinese medicine (TCM) in the treatment of MASH-related diseases.
HRMS. Molecular docking analyses confirmed that Levistilide A, a compound derived from As, exhibits strong binding affinity with multiple key targets involved in various signaling pathways, including fat metabolism, anti-inflammatory responses, and anti-apoptotic mechanisms. Several bioactive compounds identified in SbG, such as Baicalein, Scutellarin, Oroxindin, and Neobaicalein, also demonstrated favorable interactions with these pathway-associated targets. These findings provide robust evidence supporting the hypothesis that the synergistic application of As-SbG exerts therapeutic effects on MASH through a multi-component, multi-pathway, and multi-target mechanism, highlighting a novel approach of traditional Chinese medicine (TCM) in the treatment of MASH-related diseases.
Conclusion
As-SbG synergistic therapy activates Nrg4 expression in adipose tissue, which specifically binds to ErbB receptors in liver tissue, thereby modulating downstream signal transduction pathways involved in fat metabolism, anti-inflammatory responses, and anti-apoptotic processes. This inter-communication between adipose and liver tissues helps restore hepatic lipid homeostasis, attenuates inflammation, and reduces hepatocyte injury, fibrosis, and apoptosis, ultimately ameliorating MASH induced by diverse dietary regimens.
当归和黄芩通过促进脂肪组织与肝脏组织的沟通,协同缓解代谢功能障碍相关的脂肪性肝炎。
背景:当归(当归,As)与黄芩(黄芩或黄芩,SbG; As-SbG)协同使用对代谢功能障碍相关性脂肪性肝炎(MASH)有显著的治疗作用。然而,其协同作用的潜在药理学机制尚未完全阐明。目的:本研究旨在评价As-SbG协同应用对不同饮食模式诱导的MASH的治疗效果,并确定其抗MASH作用的具体潜在活性化合物、潜在药理机制和关键治疗靶点。方法:建立蛋氨酸和胆碱缺乏(MCD)和高脂/高胆固醇(HFHC)饲料诱导的MASH动物模型,比较As、SbG和As-SbG协同治疗对MASH进展过程中脂肪组织和肝脏组织的影响。通过蛋白质组学分析确定了潜在的机制途径和关键分子靶点。采用超高效液相色谱-高分辨质谱(UPLC-HRMS)对As-SbG的化学成分进行了表征。利用分子对接方法进一步验证了鉴定的生物活性成分与蛋白质组学的关键靶点之间的相互作用,重点研究了As-SbG的治疗潜力。基于这些生物信息学发现,我们采用qRT-PCR、免疫荧光染色、Western blotting等方法,阐明As-SbG联合治疗对体外和体内MASH模型中脂质代谢、炎症和凋亡相关通路的调控机制。结果:与单味给药As和SbG相比,As-SbG协同给药显著恢复了不同饮食条件下mash诱导小鼠各种脂肪组织不同表型、功能和大小之间的平衡,改善了肝脂肪变性和肝损伤。蛋白质组学分析显示,As-SbG对MASH的潜在协同治疗作用主要与脂肪代谢、抗炎反应和抗凋亡机制相关。机制探索发现As-SbG协同治疗可增强脂肪组织中Nrg4的表达,并特异性结合肝组织中的ErbB受体。这种相互作用激活了下游的PI3K/Akt、Ras/MAPK和P53信号通路,从而促进脂肪和肝组织之间的组织间通讯。因此,这种调节作用改善了肝脏脂质代谢,抑制了炎症反应,减少了肝细胞损伤、纤维化和凋亡,最终改善了MASH。此外,体外对关键靶点ErbB4的药理抑制使As-SbG协同治疗对MASH的保护作用失效。此外,利用UPLCHRMS鉴定了As-SbG的31种化学成分。分子对接分析证实,来自As的化合物Levistilide A与多个关键靶点具有很强的结合亲和力,涉及多种信号通路,包括脂肪代谢、抗炎反应和抗凋亡机制。在SbG中发现的几种生物活性化合物,如黄芩苷、黄芩苷、黄芩苷和新黄芩苷,也显示出与这些途径相关靶点的良好相互作用。这些发现有力地支持了As-SbG协同应用对MASH的多组分、多途径、多靶点机制发挥治疗作用的假设,突出了中医治疗MASH相关疾病的新途径。结论:As-SbG协同治疗激活脂肪组织中Nrg4的表达,特异结合肝组织ErbB受体,从而调节下游参与脂肪代谢、抗炎反应和抗凋亡过程的信号转导通路。脂肪和肝组织之间的这种相互交流有助于恢复肝脂质稳态,减轻炎症,减少肝细胞损伤,纤维化和凋亡,最终改善由不同饮食方案引起的MASH。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Phytomedicine
医学-药学
CiteScore
10.30
自引率
5.10%
发文量
670
审稿时长
91 days
期刊介绍:
Phytomedicine is a therapy-oriented journal that publishes innovative studies on the efficacy, safety, quality, and mechanisms of action of specified plant extracts, phytopharmaceuticals, and their isolated constituents. This includes clinical, pharmacological, pharmacokinetic, and toxicological studies of herbal medicinal products, preparations, and purified compounds with defined and consistent quality, ensuring reproducible pharmacological activity. Founded in 1994, Phytomedicine aims to focus and stimulate research in this field and establish internationally accepted scientific standards for pharmacological studies, proof of clinical efficacy, and safety of phytomedicines.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


