Euchrenone A10 attenuates septic lung injury though S100A8/A9-dependent TLR4/MyD88/NF-κB signaling
IF 8.3
1区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
Abstract
Background
Euchrenone A10 (A10), an isoprenylated flavanone isolated from Glycyrrhiza (licorice), exhibits significant bioactivities, including anti-inflammatory and antioxidant effects. However, the effects and mechanisms underlying A10′s protective role in sepsis-associated acute lung injury (SALI) remain incompletely understood.
Objective
This study aims to elucidate the pharmacological effects of A10 and the underlying mechanisms by which it protects against SALI, using both in vitro and in vivo experiments.
Methods
To evaluate the effects of A10 against SALI, mice were pretreated with A10 (12.5, 25, or 50 mg/kg) or dexamethasone (Dex, 50 μg/kg) prior to sepsis induction via intraperitoneal administration of lipopolysaccharide (LPS, 10 mg/kg) or cecal ligation and puncture (CLP). Survival rates, pulmwasy function, bronchoalveolar lavage fluid inflammatory cell infiltration, protein exudation, and lung histopathology were systematically assessed. Molecular docking and biolayer interferometry (BLI) were employed to characterize the interactions between S100A8/A9 and A10 or paquinimod (paq). Complementary in vitro studies using LPS (150 ng/ml)-stimulated Raw264.7 macrophages were conducted to examine A10′s effects on inflammatory gene expression.
Results
To evaluate the therapeutic effects of A10, we employed LPS-induced SALI and CLP-induced ALI models. A10 dose-dependently alleviated pulmonary injury and improved survival rates in septic mice. Notably, A10 inhibited the expression of S100A8/A9 and suppressed the TLR4/MyD88/NF-κB signaling pathway in both in vivo and in vitro models. Mechanistic studies using molecular docking and BLI indicated that A10 directly binds to S100A8/A9, thereby blocking its interaction with the TLR4 receptor. Furthermore, in vivo and in vitro experiments confirmed that A10 shares the same binding site on S100A8/A9 as the S100A9-specific inhibitor paq, competitively displacing paq and inhibiting downstream TLR4/MyD88/NF-κB signaling.
Conclusion
A10 exerts its anti-inflammatory effects by binding to the S100A8/A9 protein, thereby inhibiting the TLR4–NF-κB inflammatory cascade. These properties highlight its therapeutic potential as monotherapy for SALI.
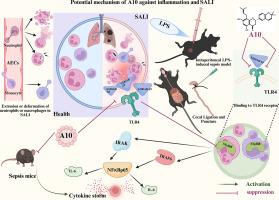
Euchrenone A10通过S100A8/ a9依赖性TLR4/MyD88/NF-κB信号通路减轻脓毒性肺损伤。
背景:Euchrenone A10 (A10)是一种从甘草中分离得到的异戊烯基黄酮,具有抗炎、抗氧化等重要生物活性。然而,A10在脓毒症相关急性肺损伤(SALI)中的保护作用及其机制尚不完全清楚。目的:本研究旨在通过体外和体内实验,阐明A10的药理作用及其预防SALI的潜在机制。方法:通过腹腔注射脂多糖(LPS, 10 mg/kg)或盲肠结扎穿刺(CLP),在脓毒症诱导前分别用A10(12.5、25、50 mg/kg)或地塞米松(Dex, 50 μg/kg)预处理小鼠,评价A10对SALI的作用。系统评估生存率、肺功能、支气管肺泡灌洗液炎症细胞浸润、蛋白渗出和肺组织病理学。采用分子对接和生物层干涉法(BLI)表征了S100A8/A9与A10或paquinimod (paq)的相互作用。补充体外实验采用LPS (150 ng/ml)刺激Raw264.7巨噬细胞,研究A10对炎症基因表达的影响。结果:采用lps诱导的SALI和clp诱导的ALI模型评价A10的治疗效果。A10剂量依赖性地减轻了脓毒症小鼠的肺损伤,提高了存活率。值得注意的是,在体内和体外模型中,A10均能抑制S100A8/A9的表达,抑制TLR4/MyD88/NF-κB信号通路。分子对接和BLI的机制研究表明,A10直接结合到S100A8/A9上,从而阻断其与TLR4受体的相互作用。此外,体内和体外实验证实,A10与S100A8/A9特异性抑制剂paq在S100A8/A9上具有相同的结合位点,竞争性地取代paq,抑制下游TLR4/MyD88/NF-κB信号通路。结论:A10通过与S100A8/A9蛋白结合,抑制TLR4-NF-κB炎症级联,发挥抗炎作用。这些特性突出了其作为SALI单一疗法的治疗潜力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Phytomedicine
医学-药学
CiteScore
10.30
自引率
5.10%
发文量
670
审稿时长
91 days
期刊介绍:
Phytomedicine is a therapy-oriented journal that publishes innovative studies on the efficacy, safety, quality, and mechanisms of action of specified plant extracts, phytopharmaceuticals, and their isolated constituents. This includes clinical, pharmacological, pharmacokinetic, and toxicological studies of herbal medicinal products, preparations, and purified compounds with defined and consistent quality, ensuring reproducible pharmacological activity. Founded in 1994, Phytomedicine aims to focus and stimulate research in this field and establish internationally accepted scientific standards for pharmacological studies, proof of clinical efficacy, and safety of phytomedicines.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


