Zhenqi Fuzheng Granule targets the SCFAs-GPR109A axis to enhance PD-1 antibody efficacy via immunometabolic remodeling in colorectal cancer
IF 8.3
1区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
Abstract
Background
Immune checkpoint inhibitors (ICIs), particularly PD-1 antibodies, represent a breakthrough in colorectal cancer (CRC) treatment. However, their clinical efficacy remains limited by tumour-induced immunosuppression. Traditional Chinese medicine (TCM) has attracted growing interest as a potential adjuvant to immunotherapy. Zhenqi Fuzheng Granule (ZQFZ) is a clinically approved herbal prescription widely used as an adjuvant therapy for CRC, yet its mechanistic underpinnings remain elusive.
Objective
To investigate how ZQFZ improves the efficacy in CRC, with emphasis on gut microbiota modulation, SCFAs production, and downstream immunometabolic pathways involving GPR109A, and confirms that butyrate plays an important role in colorectal cancer inhibition.
Methods
Phytochemical analysis of ZQFZ was conducted using LC-MS/MS and UPLC-MS/MS, identifying and quantifying seven major compounds. In vivo experiments, AOM/DSS-induced CRC mouse models were treated with ZQFZ, PD-1 antibody, or their combination. Tumour progression, body weight, and survival were monitored. Gut microbial composition and colonic SCFAs levels were assessed via 16S rRNA sequencing and gas chromatography. RT-qPCR was employed to validate the expression of key genes associated with the GPR109A/AKT/mTOR/HIF-1α signaling pathway. Molecular changes in the GPR109A/AKT/mTOR/HIF-1α pathway were evaluated through Western blotting, transcriptomic, and proteomic analyses. Immune cell infiltration and phenotypes were analyzed by flow cytometry. Molecular docking and molecular dynamics simulations were conducted to predict the binding affinity and structural stability between GPR109A and AKT1. The interactions between GPR109A and AKT1, as well as between butyrate and GPR109A, were further validated in vitro using microscale thermophoresis (MST) assays. To evaluate the microbial basis of ZQFZ activity, antibiotic-pretreated mice received ZQFZ-derived fecal microbiota transplantation (FMT). In vitro experiments, to investigate the mechanism by which sodium butyrate (NaB), the major gut microbial metabolite of ZQFZ, inhibits glycolysis in colorectal cancer under hypoxic conditions, CCK-8 assays, flow cytometry, lactate measurements, and Western blotting were performed to assess cell viability, apoptosis, lactate production, and the expression of AKT/mTOR/HIF-1α and glycolysis-related proteins.
Results
LC-MS/MS profiling identified multiple bioactive constituents in ZQFZ. Targeted UPLC-MS/MS quantification revealed that the formulation contained Adenosine (0.87mg/g), Salidroside (0.11 mg/g), Astragaloside IV (0.07 mg/g), Calycosin (0.03 mg/g), Formononetin (6.7 μg /g), Chlorogenic acid (1.4 μg/g), Apigenin (0.5 μg/g). In vivo studies, both ZQFZ and PD-1 antibody inhibited tumour growth, with the combination treatment exerting the most pronounced antitumour effects. ZQFZ reshaped the gut microbiota, increased the levels of short-chain fatty acids (SCFAs), particularly butyrate, and activated the GPR109A pathway, leading to downregulation of the AKT/mTOR/HIF-1α signaling axis, suppression of HK2 expression and lactate production, and consequent inhibition of glycolysis. Immune remodeling was also observed, including reduced infiltration of myeloid-derived suppressor cells (MDSCs), polarization of macrophages toward the M1 phenotype, restoration of the CD4⁺/CD8⁺ T cell ratio, and modulation of serum cytokines including upregulation of IL-2, IL-12, and IFN-γ, along with downregulation of IL-4 and IL-10. ZQFZ-derived FMT significantly inhibited tumour growth, suppressed glycolysis-related markers (PKM2, GLUT1, HIF-1α, LDHA), and remodeled the immune microenvironment by reducing MDSCs and enhancing M1 macrophages and CD8⁺ T cell infiltration. In hypoxia-mimicking in vitro experiments, sodium butyrate (NaB), the principal gut microbial metabolite of ZQFZ, suppressed colorectal cancer cell viability and induced apoptosis. Through activation of GPR109A, NaB inhibited the AKT/mTOR/HIF-1α pathway and glycolysis-related enzymes, reduced lactate production, and further suppressed glycolysis. Molecular docking and dynamics simulations suggested a stable interaction between GPR109A and AKT1, which was confirmed in vitro by MST showing high-affinity binding (Kd=74.5 ± 20.8 nM); MST also verified moderate-affinity binding between GPR109A and sodium butyrate (Kd=43.3 ± 6.5 μM), supporting a dual interaction model wherein butyrate activates GPR109A, which in turn directly binds AKT1 to inhibit downstream glycolytic signaling.
Conclusion
This study uncovers a novel integrated mechanism whereby ZQFZ enhances PD-1 antibody efficacy via the gut microbiota-SCFAs-GPR109A axis, and NaB-mediated glycolysis inhibition under hypoxia further confirms its immunometabolic mechanism against CRC.
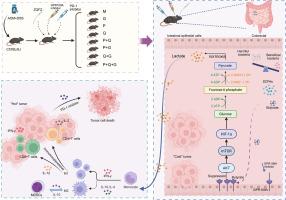
贞芪扶正颗粒靶向SCFAs-GPR109A轴,通过免疫代谢重塑增强结直肠癌PD-1抗体的疗效。
背景:免疫检查点抑制剂(ICIs),特别是PD-1抗体,代表了结直肠癌(CRC)治疗的突破。然而,它们的临床疗效仍然受到肿瘤诱导的免疫抑制的限制。中药作为一种潜在的免疫辅助疗法引起了人们越来越多的兴趣。贞芪扶正颗粒(ZQFZ)是一种临床批准的中草药处方,广泛用于结直肠癌的辅助治疗,但其机制基础尚不清楚。目的:探讨ZQFZ如何提高结直肠癌的疗效,重点研究肠道菌群调节、SCFAs产生以及GPR109A参与的下游免疫代谢途径,证实丁酸盐在结直肠癌抑制中发挥重要作用。方法:采用LC-MS/MS和UPLC-MS/MS对ZQFZ进行植物化学分析,对7个主要化合物进行鉴定和定量。在体内实验中,采用ZQFZ、PD-1抗体或两者联合治疗AOM/ dss诱导的结直肠癌小鼠模型。监测肿瘤进展、体重和生存。通过16S rRNA测序和气相色谱法评估肠道微生物组成和结肠SCFAs水平。采用RT-qPCR验证GPR109A/AKT/mTOR/HIF-1α信号通路相关关键基因的表达。通过Western blotting、转录组学和蛋白质组学分析评估GPR109A/AKT/mTOR/HIF-1α通路的分子变化。流式细胞术分析免疫细胞浸润及表型。通过分子对接和分子动力学模拟,预测GPR109A与AKT1的结合亲和力和结构稳定性。GPR109A与AKT1、丁酸盐与GPR109A之间的相互作用通过体外微尺度热泳(MST)实验进一步验证。为了评估ZQFZ活性的微生物基础,抗生素预处理的小鼠接受ZQFZ来源的粪便微生物群移植(FMT)。体外实验中,为了研究ZQFZ的主要肠道微生物代谢物丁酸钠(NaB)在低氧条件下抑制结直肠癌糖酵解的机制,我们采用CCK-8测定、流式细胞术、乳酸测定和Western blotting来评估细胞活力、凋亡、乳酸生成以及AKT/mTOR/HIF-1α和糖酵解相关蛋白的表达。结果:hplc -MS/MS图谱分析鉴定出ZQFZ中多种生物活性成分。靶向UPLC-MS/MS定量分析结果显示:该制剂中含有腺苷(0.87mg/g)、红景天苷(0.11 mg/g)、黄芪甲苷(0.07 mg/g)、毛蕊花素(0.03 mg/g)、芒柄花素(6.7 μg)、绿原酸(1.4 μg/g)、芹菜素(0.5 μg/g)。在体内研究中,ZQFZ和PD-1抗体均能抑制肿瘤生长,其中联合治疗的抗肿瘤效果最为显著。ZQFZ重塑肠道菌群,增加短链脂肪酸(SCFAs)水平,特别是丁酸盐,激活GPR109A通路,导致AKT/mTOR/HIF-1α信号轴下调,抑制HK2表达和乳酸生成,进而抑制糖酵解。还观察到免疫重塑,包括髓源性抑制细胞(MDSCs)浸润减少,巨噬细胞向M1表型极化,CD4 + /CD8 + T细胞比例恢复,血清细胞因子调节,包括IL-2、IL-12和IFN-γ上调,以及IL-4和IL-10下调。zqfz衍生的FMT显著抑制肿瘤生长,抑制糖酵解相关标志物(PKM2、GLUT1、HIF-1α、LDHA),并通过减少MDSCs、增强M1巨噬细胞和CD8 + T细胞浸润来重塑免疫微环境。在体外模拟缺氧实验中,ZQFZ的主要肠道微生物代谢物丁酸钠(NaB)抑制结直肠癌细胞活力并诱导凋亡。NaB通过激活GPR109A,抑制AKT/mTOR/HIF-1α通路和糖酵解相关酶,降低乳酸生成,进一步抑制糖酵解。分子对接和动力学模拟表明,GPR109A与AKT1具有稳定的相互作用,体外MST证实了这一结果,显示出高亲和力结合(Kd=74.5±20.8 nM);MST还验证了GPR109A与丁酸钠之间的中等亲和力结合(Kd=43.3±6.5 μM),支持丁酸钠激活GPR109A的双重相互作用模型,GPR109A反过来直接结合AKT1抑制下游糖酵解信号。结论:本研究揭示了ZQFZ通过肠道菌群- scfas - gpr109a轴增强PD-1抗体效能的全新整合机制,nab介导的缺氧条件下糖酵解抑制进一步证实了其抗CRC的免疫代谢机制。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Phytomedicine
医学-药学
CiteScore
10.30
自引率
5.10%
发文量
670
审稿时长
91 days
期刊介绍:
Phytomedicine is a therapy-oriented journal that publishes innovative studies on the efficacy, safety, quality, and mechanisms of action of specified plant extracts, phytopharmaceuticals, and their isolated constituents. This includes clinical, pharmacological, pharmacokinetic, and toxicological studies of herbal medicinal products, preparations, and purified compounds with defined and consistent quality, ensuring reproducible pharmacological activity. Founded in 1994, Phytomedicine aims to focus and stimulate research in this field and establish internationally accepted scientific standards for pharmacological studies, proof of clinical efficacy, and safety of phytomedicines.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


