Early results from implementation of HIV pre-exposure prophylaxis (PrEP) with long-acting injectable cabotegravir for people with barriers to oral strategies, Italy, December 2024 to August 2025.
IF 7.8
2区 医学
Q1 INFECTIOUS DISEASES
引用次数: 0
Abstract
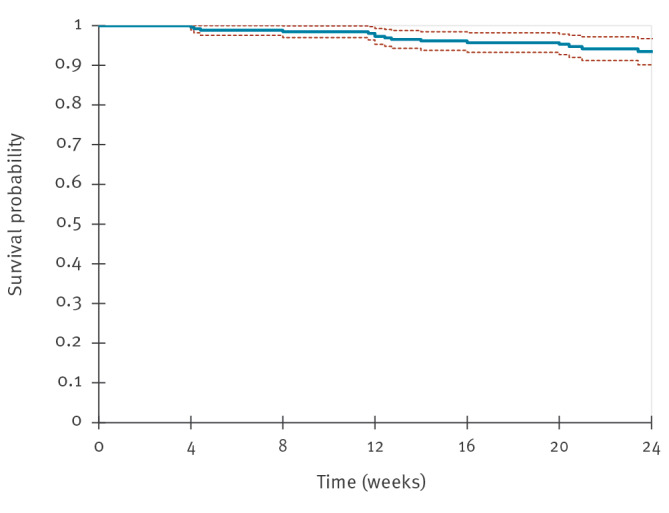
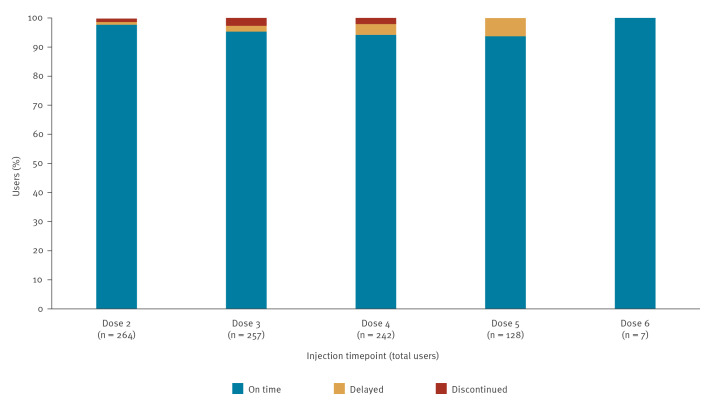
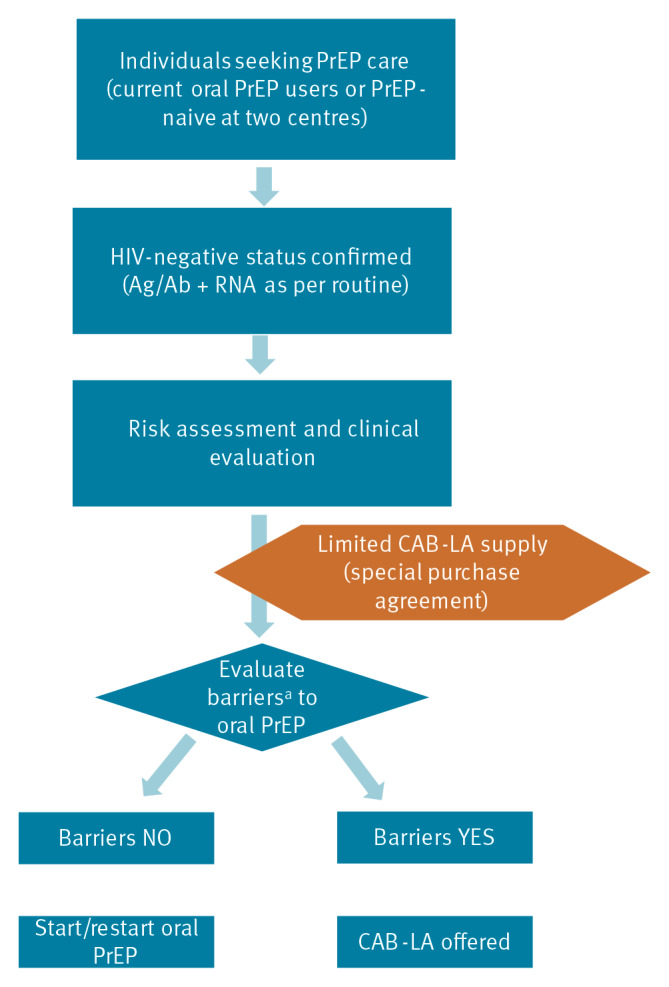
Between December 2024 and August 2025, two Italian HIV/STI clinics offered long-acting injectable cabotegravir (CAB-LA) for HIV prevention in 265 individuals, representing the first European real-world implementation. Participants were prioritised based on vulnerabilities compromising oral pre-exposure prophylaxis (PrEP), such as poor adherence, comorbidities and behavioural barriers. Adherence to injections exceeded 95%, tolerability was favourable and only 1.5% discontinued for drug-related reasons. These findings demonstrate the feasibility of CAB-LA in European clinical services and support its use in specific populations.
2024年12月至2025年8月,意大利对口服策略有障碍的人群实施长效可注射卡波特韦的HIV暴露前预防(PrEP)的早期结果。
在2024年12月至2025年8月期间,意大利的两家艾滋病毒/性传播感染诊所为265人提供了长效注射卡波特韦(CAB-LA),这是欧洲首次在现实世界中实施。根据影响口服暴露前预防(PrEP)的脆弱性(如依从性差、合并症和行为障碍)对参与者进行优先排序。注射依从性超过95%,耐受性良好,只有1.5%因药物相关原因停药。这些发现证明了CAB-LA在欧洲临床服务中的可行性,并支持其在特定人群中的应用。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Eurosurveillance
INFECTIOUS DISEASES-
CiteScore
32.70
自引率
2.10%
发文量
430
审稿时长
3-8 weeks
期刊介绍:
Eurosurveillance is a European peer-reviewed journal focusing on the epidemiology, surveillance, prevention, and control of communicable diseases relevant to Europe.It is a weekly online journal, with 50 issues per year published on Thursdays. The journal includes short rapid communications, in-depth research articles, surveillance reports, reviews, and perspective papers. It excels in timely publication of authoritative papers on ongoing outbreaks or other public health events. Under special circumstances when current events need to be urgently communicated to readers for rapid public health action, e-alerts can be released outside of the regular publishing schedule. Additionally, topical compilations and special issues may be provided in PDF format.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


