Single-cell atlas of cervical organoids uncovers epithelial immune heterogeneity and intercellular cross-talk during Chlamydia infection
IF 12.5
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
Abstract
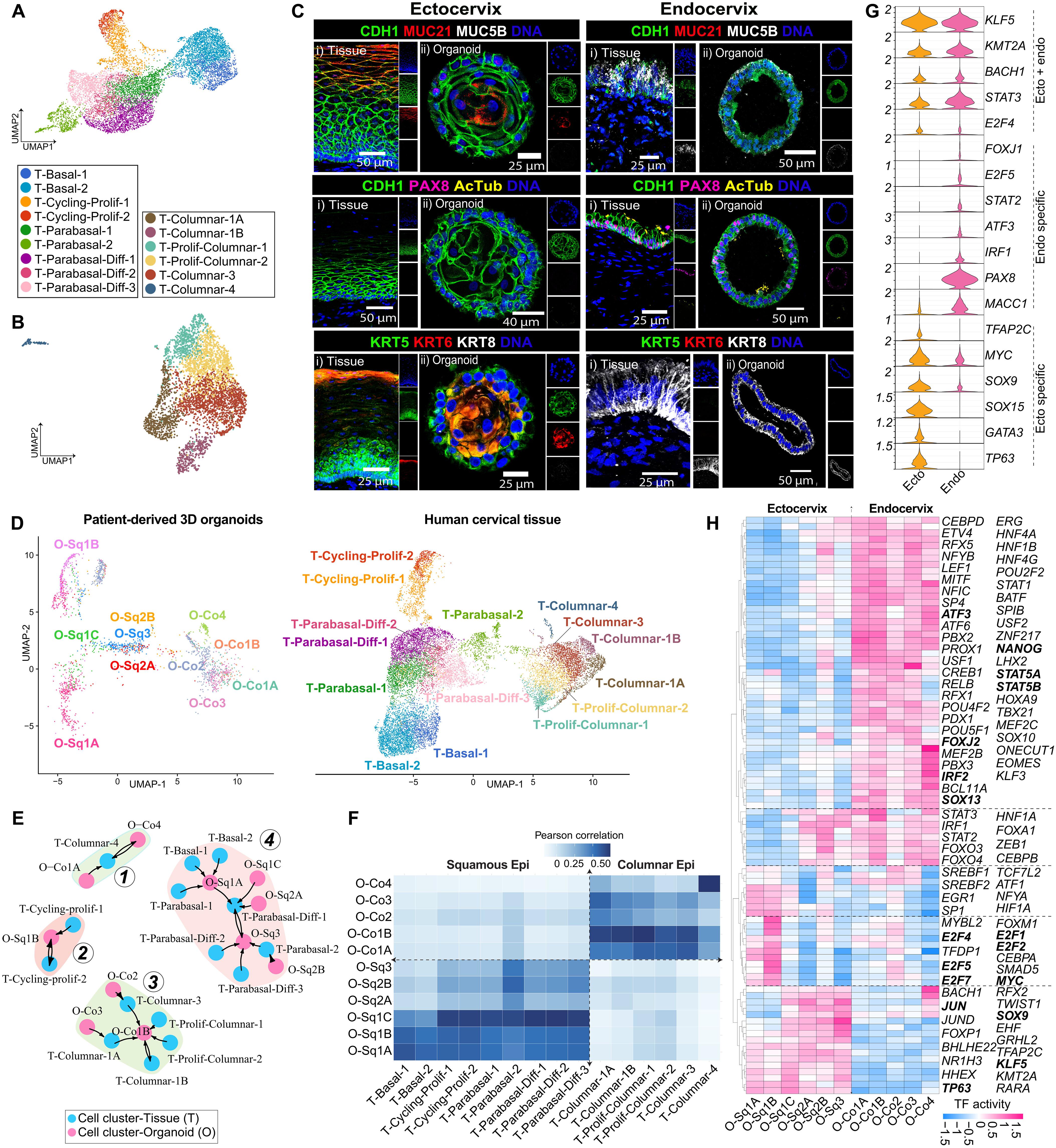
The uterine cervix is a critical mucosal interface that balances immune defense and reproductive function, yet how its distinct epithelial compartments coordinate responses to infection remains unclear. Here, we integrate patient-derived three-dimensional cervical organoids, single-cell transcriptomics, and native tissue analysis to construct a high-resolution atlas of epithelial cell diversity and immune dynamics during Chlamydia trachomatis infection. We demonstrate that cervical organoids closely resemble native tissue at transcriptional and cellular levels, identifying epithelial subtypes with region-specific immune specializations. Upon infection, ectocervical epithelia reinforce barrier integrity, whereas endocervical epithelia, particularly uninfected bystander cells, exhibit extensive transcriptional reprogramming characterized by robust interferon activation, antigen presentation, and antimicrobial defense. Infection profoundly reshapes epithelial intercellular communication, positioning bystander cells as major contributors to signaling networks coordinating immune responses and tissue regeneration. Our findings highlight a sophisticated epithelial-intrinsic immune network critical for cervical mucosal defense and establish a physiologically relevant platform for studying host-pathogen interactions and guiding targeted mucosal therapies against reproductive tract infections and pathologies.
子宫颈类器官单细胞图谱揭示衣原体感染期间上皮免疫异质性和细胞间串扰
子宫颈是平衡免疫防御和生殖功能的关键粘膜界面,但其独特的上皮隔室如何协调对感染的反应尚不清楚。在这里,我们整合了患者来源的三维宫颈类器官,单细胞转录组学和天然组织分析,以构建沙眼衣原体感染期间上皮细胞多样性和免疫动力学的高分辨率图谱。我们证明,宫颈类器官在转录和细胞水平上与天然组织非常相似,识别出具有区域特异性免疫特化的上皮亚型。感染后,宫颈外上皮加强屏障的完整性,而宫颈内上皮,特别是未感染的旁观者细胞,表现出广泛的转录重编程,其特征是强大的干扰素激活、抗原呈递和抗菌防御。感染深刻地重塑了上皮细胞间的通讯,将旁观者细胞定位为协调免疫反应和组织再生的信号网络的主要贡献者。我们的研究结果强调了一个复杂的上皮-内在免疫网络对宫颈粘膜防御至关重要,并为研究宿主-病原体相互作用和指导针对生殖道感染和病理的靶向粘膜治疗建立了生理学相关平台。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Science Advances
综合性期刊-综合性期刊
CiteScore
21.40
自引率
1.50%
发文量
1937
审稿时长
29 weeks
期刊介绍:
Science Advances, an open-access journal by AAAS, publishes impactful research in diverse scientific areas. It aims for fair, fast, and expert peer review, providing freely accessible research to readers. Led by distinguished scientists, the journal supports AAAS's mission by extending Science magazine's capacity to identify and promote significant advances. Evolving digital publishing technologies play a crucial role in advancing AAAS's global mission for science communication and benefitting humankind.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


