Mechanochemical waves in focal adhesions during cell migration
IF 12.5
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
Abstract
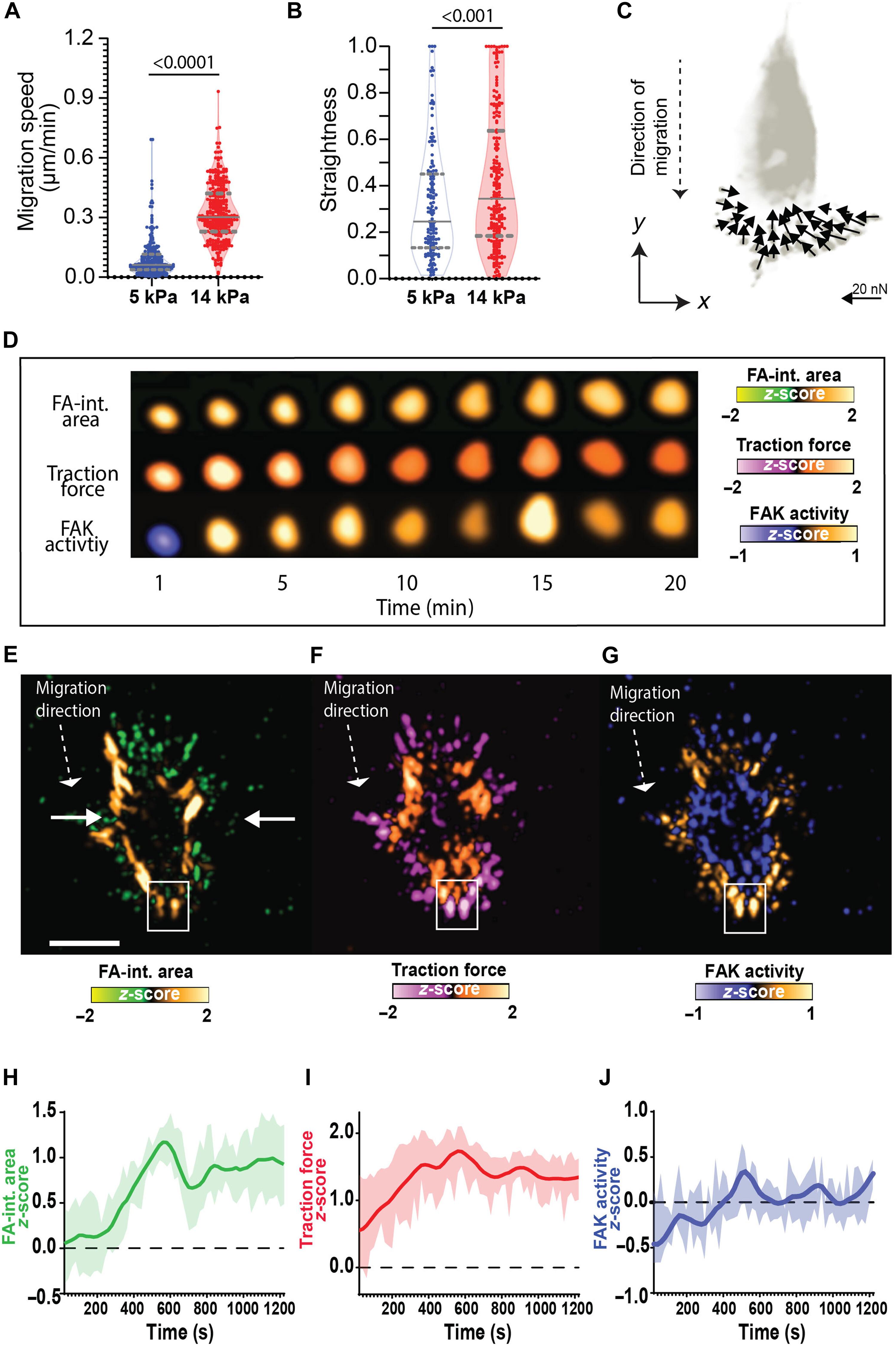
Focal adhesions (FAs) are dynamic structures central to cell migration, serving as mechanotransduction sites linking the extracellular matrix (ECM) to intracellular signaling pathways such as FA kinase (FAK). How FAK becomes activated in response to cell-ECM adhesive forces at single FAs to facilitate directional motion is poorly understood. Using micropillar-based force microscopy and FA-targeted FRET biosensors, we monitored real-time traction forces and FAK activity at individual FAs during assembly and disassembly. Our results demonstrate oscillatory temporal coupling of traction force and FAK activity in high-tension FAs before FA disassembly. Cross-correlation analyses revealed that force precedes FAK activation, guiding FA turnover. Atomistic molecular simulations unveiled a force-induced mechanism where traction forces disrupt autoinhibitory FERM-kinase interactions in FAK, enabling catalytic activity without structural unfolding. Our findings provide mechanistic insights into the spatiotemporal integration of mechanical forces and biochemical signaling in cell migration.
细胞迁移过程中局灶黏附中的机械化学波
局灶黏附(FAs)是细胞迁移的核心动态结构,作为连接细胞外基质(ECM)和细胞内信号通路(如FA激酶(FAK))的机械转导位点。FAK如何响应细胞- ecm在单个FAs上的粘附力而被激活,以促进定向运动,目前尚不清楚。利用基于微柱的力显微镜和fa靶向FRET生物传感器,我们监测了装配和拆卸过程中单个fa的实时牵引力和FAK活性。我们的研究结果表明,在FA拆卸之前,高张力FA的牵引力和FAK活性存在振荡时间耦合。交叉相关分析显示,力先于FAK激活,引导FA转换。原子分子模拟揭示了一种力诱导机制,其中牵引力破坏FAK中的自抑制ferm -激酶相互作用,使催化活性无需结构展开。我们的发现为细胞迁移中机械力和生化信号的时空整合提供了机制上的见解。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Science Advances
综合性期刊-综合性期刊
CiteScore
21.40
自引率
1.50%
发文量
1937
审稿时长
29 weeks
期刊介绍:
Science Advances, an open-access journal by AAAS, publishes impactful research in diverse scientific areas. It aims for fair, fast, and expert peer review, providing freely accessible research to readers. Led by distinguished scientists, the journal supports AAAS's mission by extending Science magazine's capacity to identify and promote significant advances. Evolving digital publishing technologies play a crucial role in advancing AAAS's global mission for science communication and benefitting humankind.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


