Secretory IgA amplification during immune checkpoint blockade enhances the control of tumor growth by enterotropic T cells
IF 12.5
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
Abstract
The gut microbiota is essential for many aspects of host physiology, and secretory immunoglobulin A (sIgA) modulates its function. The microbiota community determines the efficacy of immune checkpoint blockade (ICB) in cancer immunotherapy; however, mechanisms able to improve this function are not known. Extracellular adenosine triphosphate (ATP) released by the microbiota restricts the sIgA repertoire by limiting T follicular helper (TFH) cell activity in the Peyer’s patches via stimulation of the ionotropic P2X7 receptor. We show that sIgA amplification by oral administration of the ATP hydrolyzing enzyme apyrase corrects enteropathic features of ICB and improves therapeutic efficacy. Consistent with sIgA function in reshaping the gut ecosystem and enhancing ICB, IgA−/− mice did not show any improvement of antitumor response by apyrase administration. Mechanistically, data in mice and patients with cancer suggest that invigorated enterotropic cytotoxic T cells expressing the chemokine receptor CCR9 replenish the tumor microenvironment in a CCL25-mediated manner and control tumor growth, resulting in improved ICB efficacy.
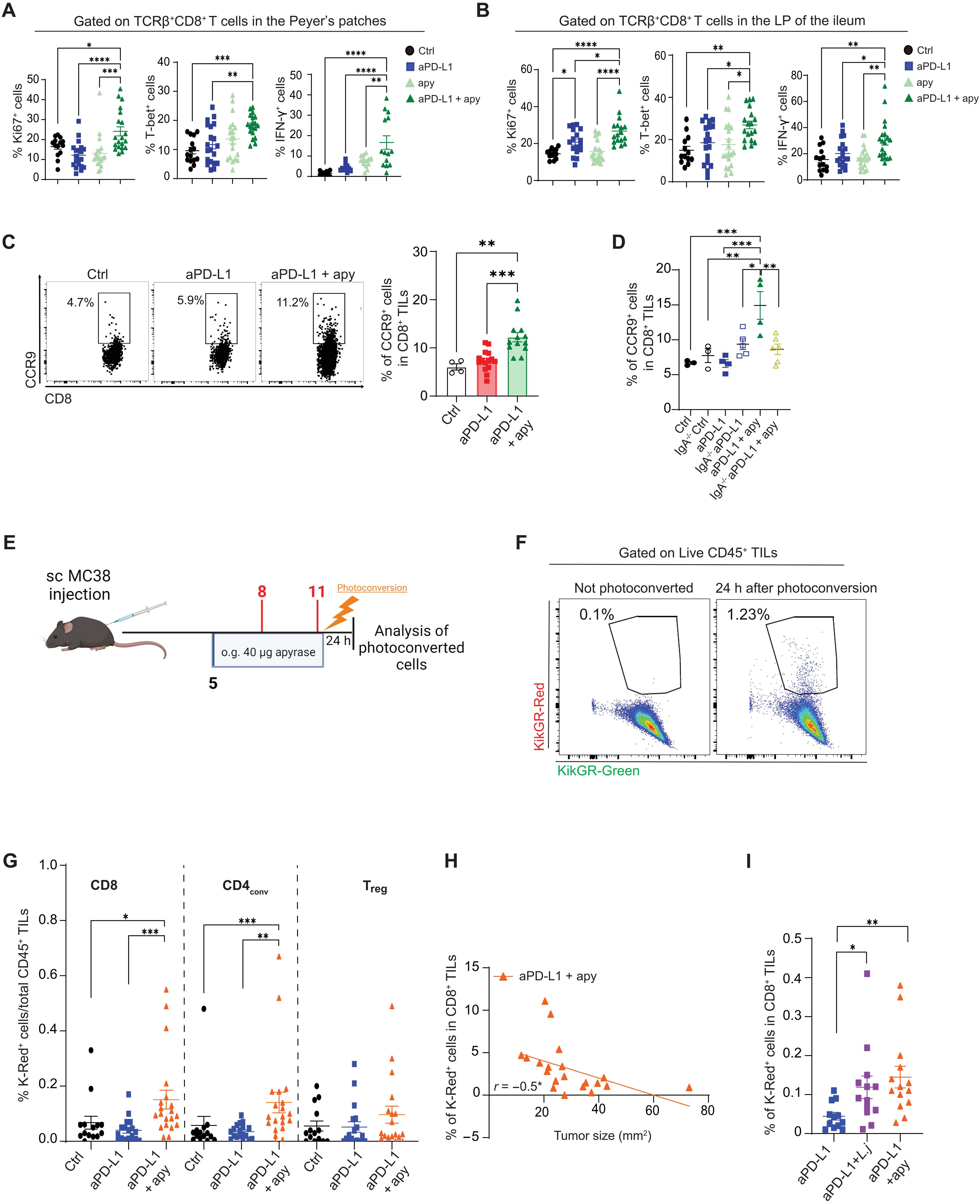
免疫检查点阻断期间分泌的IgA扩增增强了嗜肠性T细胞对肿瘤生长的控制
肠道微生物群对宿主生理的许多方面都是必不可少的,而分泌免疫球蛋白A (sIgA)调节其功能。微生物群落决定了免疫检查点阻断(ICB)在癌症免疫治疗中的疗效;然而,能够改善这一功能的机制尚不清楚。微生物群释放的细胞外三磷酸腺苷(ATP)通过刺激嗜电性P2X7受体,限制了Peyer斑块中T滤泡辅助(tfh)细胞的活性,从而限制了sIgA库。我们发现,通过口服ATP水解酶apyrase来扩增sIgA,可以纠正ICB的肠病特征,提高治疗效果。与sIgA重塑肠道生态系统和增强ICB的功能一致,IgA - / -小鼠没有显示出通过apyrase给予抗肿瘤反应的任何改善。在机制上,小鼠和癌症患者的数据表明,表达趋化因子受体CCR9的促肠性细胞毒性T细胞以ccl25介导的方式补充肿瘤微环境,控制肿瘤生长,从而提高ICB的疗效。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Science Advances
综合性期刊-综合性期刊
CiteScore
21.40
自引率
1.50%
发文量
1937
审稿时长
29 weeks
期刊介绍:
Science Advances, an open-access journal by AAAS, publishes impactful research in diverse scientific areas. It aims for fair, fast, and expert peer review, providing freely accessible research to readers. Led by distinguished scientists, the journal supports AAAS's mission by extending Science magazine's capacity to identify and promote significant advances. Evolving digital publishing technologies play a crucial role in advancing AAAS's global mission for science communication and benefitting humankind.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


