Epoxide Alcoholysis over M-BEA Zeolites: Effects of Alcohol Chain Length on Rates and Regioselectivities
IF 13.1
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
The structures of nucleophilic reactants affect their coordination behavior among solvent molecules and kinetics of reactions with surface intermediates within the confines of fluid-filled pores of zeolites and other microporous materials. Consequently, rates and regioselectivities of diverse chemistries may depend sensitively on nucleophile identity in manners not observed for classic fluid phase reactions. Here, we examine the impact of varying the primary alcohol (ROH) chain length on the kinetics of 1,2-epoxybutane (C4H8O) ring-opening within Brønsted (Al-BEA) and Lewis acid (Zr-BEA) zeolites. Turnover rates increase by factors of ∼6 (Al-BEA) and 4-fold (Zr-BEA) between methanol and 1-hexanol, yet the reaction mechanisms remain comparable. Despite modest rate differences, apparent activation enthalpies calculated from rates and activities of solvated reactants decrease linearly by 12 (Al-BEA) to 33 kJ mol–1 (Zr-BEA) with increased proton affinity, which suggests bond formation energies for the nucleophile strongly influence rate increases. The molecular interpretation of these trends demonstrates, however, that the solvation of ring-opening transition states by zeolite pore structures and solvent molecules also governs rates. The impact of local solvating interactions appears most directly as changes in regioselectivities, which tend to enhance terminal alcohol formation with increasing ROH chain length. Regioselectivities largely do not vary with differences in fluid composition for a given ROH. The addition of H2O increases the number of hydrogen bonds among reactive species, and trends in regioselectivities imply that the decreased hydrogen bonding ability of longer chain ROH, and not the nucleophile strength or steric bulk, determines the regioselectivities of the resulting products. This work provides direct experimental evidence that nucleophilicity and hydrogen bonding influence reaction barriers and regioselectivities in zeolite-catalyzed epoxide ring-opening, offering pathways to better control reaction kinetics.
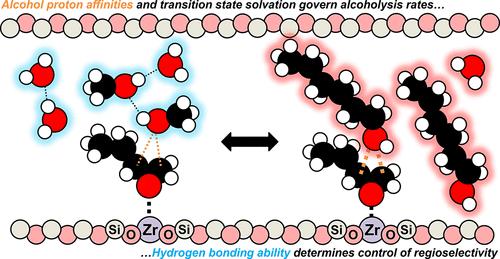
M-BEA沸石上的环氧醇解:醇链长度对速率和区域选择性的影响
亲核反应物的结构影响其与溶剂分子的配位行为以及与表面中间体在沸石和其他微孔材料的充液孔内的反应动力学。因此,不同化学反应的速率和区域选择性可能敏感地依赖于亲核试剂的同一性,这在经典的液相反应中是没有观察到的。在这里,我们研究了不同的伯醇(ROH)链长对Brønsted (Al-BEA)和Lewis酸(Zr-BEA)沸石中1,2-环氧丁烷(c4h80)开环动力学的影响。甲醇和1-己醇之间的周转率增加了约6倍(Al-BEA)和4倍(Zr-BEA),但反应机制仍然相似。尽管速率差异不大,但根据溶剂化反应物的速率和活度计算的表观活化焓随着质子亲和性的增加而线性降低12 (Al-BEA)至33 kJ mol-1 (Zr-BEA),这表明亲核试剂的成键能强烈影响速率的增加。然而,这些趋势的分子解释表明,沸石孔结构和溶剂分子对开环过渡态的溶剂化也决定了速率。局部溶剂化作用的影响最直接表现为区域选择性的变化,随着ROH链长度的增加,区域选择性倾向于促进末端醇的形成。对于给定的ROH,区域选择性基本上不随流体成分的不同而变化。H2O的加入增加了反应物质之间氢键的数量,区域选择性的趋势表明,决定产物区域选择性的不是亲核试剂的强度或空间体积,而是长链ROH的氢键能力的降低。这项工作提供了直接的实验证据,证明亲核性和氢键影响沸石催化环氧化物开环的反应屏障和区域选择性,为更好地控制反应动力学提供了途径。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Catalysis
CHEMISTRY, PHYSICAL-
CiteScore
20.80
自引率
6.20%
发文量
1253
审稿时长
1.5 months
期刊介绍:
ACS Catalysis is an esteemed journal that publishes original research in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. It offers broad coverage across diverse areas such as life sciences, organometallics and synthesis, photochemistry and electrochemistry, drug discovery and synthesis, materials science, environmental protection, polymer discovery and synthesis, and energy and fuels.
The scope of the journal is to showcase innovative work in various aspects of catalysis. This includes new reactions and novel synthetic approaches utilizing known catalysts, the discovery or modification of new catalysts, elucidation of catalytic mechanisms through cutting-edge investigations, practical enhancements of existing processes, as well as conceptual advances in the field. Contributions to ACS Catalysis can encompass both experimental and theoretical research focused on catalytic molecules, macromolecules, and materials that exhibit catalytic turnover.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


