Million-Fold Activation of C–H Bonds by Fluorinated Nonheme FeIV═O Complexes via Second Sphere Equatorial Substitution and Catalytic Epoxidation to Boot
IF 13.1
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
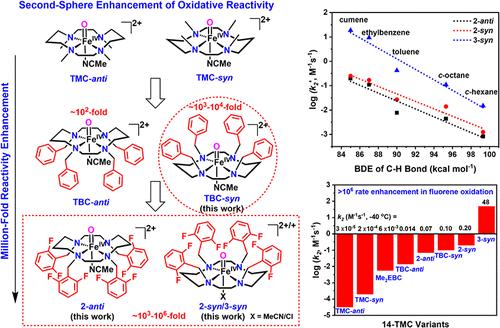
FeIV═O units found in the active sites of nonheme iron oxygenases and related synthetic analogs are intriguing intermediates capable of performing challenging oxygenation reactions. The first crystal structure of such a crucial species in a synthetic complex, [FeIV(Oanti)(TMC)(MeCN)]2+ (TMC-anti), reported in 2003, utilizes a 14-TMC (tetramethylcyclam) N4-macrocyclic ligand. With a half-life of 10 h at 25 °C, TMC-anti is quite a sluggish oxidant, but axial ligand replacements enhance TMC-anti reactivity by as much as 50-fold. Herein we switch to an N4-equatorial modification approach by replacing the N-methyl groups in TMC-anti with N–CH2–aryl groups and fluorinated analogs in the secondary coordination sphere to generate even more reactive FeIV(O)L complexes, namely [FeIV(Oanti)(TBF8C)(MeCN)]2+ (2-anti, t1/2 = 6 min at 25 °C), [FeIV(Osyn)(TBF8C)(MeCN)]2+ (2-syn, t1/2 = 2 min at 25 °C) and [FeIV(Osyn)(TBF8C)(Cl)]+ (3-syn, t1/2 = 1.5 min at −20 °C). Surprisingly, despite the increased steric bulk introduced around the FeIV═O moiety, 2-syn and 3-syn exhibit reaction rates as much as a million-fold higher than TMC-anti in C–H bond cleavage as well as oxo-transfer reactions, including unprecedented catalytic epoxidation of olefins by 2-syn. Computations confirm the dramatic reactivity enhancement upon introduction of polyfluorinated arenes into the second coordination sphere of the nonheme FeIV═O complexes, which distort the Me4cyclam that decreases the energy gap between the ground S = 1 and the excited S = 2 spin states.
氟化非血红素FeIV = O配合物通过二次球赤道取代和催化环氧化反应活化碳氢键的百万倍研究
在非血红素铁加氧酶的活性位点和相关的合成类似物中发现的FeIV = O单位是有趣的中间体,能够进行具有挑战性的氧合反应。2003年报道的合成配合物中这种关键物质的第一个晶体结构[FeIV(Oanti)(TMC)(MeCN)]2+ (TMC-anti)利用了14-TMC(四甲基环)n4大环配体。TMC-anti在25°C下的半衰期为10 h,是一种相当迟钝的氧化剂,但轴向配体的替换使TMC-anti的反应性提高了50倍。本文采用n4 -平伏修饰方法,将TMC-anti中的n -甲基替换为二级配位球中的n - ch2 -芳基和氟化类似物,生成更具活性的FeIV(O)L配合物,即[FeIV(Oanti)(TBF8C)(MeCN)]2+ (2-anti, t1/2 = 6 min, 25℃),[FeIV(Osyn)(TBF8C)(MeCN)]2+ (2-syn, t1/2 = 2 min, 25℃)和[FeIV(Osyn)(TBF8C)(Cl)]+ (3-syn, t1/2 = 1.5 min, - 20℃)。令人惊讶的是,尽管在FeIV = O部分周围引入了更多的立体体积,2-syn和3-syn在C-H键裂解和氧转移反应中表现出比TMC-anti高一百万倍的反应速率,包括2-syn对烯烃的前所未有的催化环氧化。计算证实,在非血红素FeIV = O配合物的第二配位球中引入多氟芳烃后,反应性显著增强,这使得Me4cyclam扭曲,减小了基态S = 1和激发态S = 2自旋态之间的能隙。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Catalysis
CHEMISTRY, PHYSICAL-
CiteScore
20.80
自引率
6.20%
发文量
1253
审稿时长
1.5 months
期刊介绍:
ACS Catalysis is an esteemed journal that publishes original research in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. It offers broad coverage across diverse areas such as life sciences, organometallics and synthesis, photochemistry and electrochemistry, drug discovery and synthesis, materials science, environmental protection, polymer discovery and synthesis, and energy and fuels.
The scope of the journal is to showcase innovative work in various aspects of catalysis. This includes new reactions and novel synthetic approaches utilizing known catalysts, the discovery or modification of new catalysts, elucidation of catalytic mechanisms through cutting-edge investigations, practical enhancements of existing processes, as well as conceptual advances in the field. Contributions to ACS Catalysis can encompass both experimental and theoretical research focused on catalytic molecules, macromolecules, and materials that exhibit catalytic turnover.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


