Modulating the Interfacial Energy of Ni–Bi Molten Alloys for Enhanced Methane Decomposition to Hydrogen
IF 13.1
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
Catalytic methane decomposition is a highly promising CO2-free hydrogen production technology with carbon material generation; however, developing catalysts that can efficiently decompose methane at moderate temperatures remains challenging. In this study, we develop a series of NiMn–Bi molten alloy catalysts with various Ni:Mn ratios for catalyzing methane decomposition. The Mn-modified Ni–Bi alloy exhibits a CH4 conversion of 15.3% at 850 °C, and the corresponding hydrogen production rate increases by 112% compared with Ni–Bi. The ternary alloy catalyst also demonstrates stability at this production rate for up to 80 h. Molecular dynamics simulations show that the introduction of Mn significantly reduces the strong interaction between the active metal Ni and the solvent metal Bi, thereby accelerating the methane dissociation rate. More importantly, among the theoretically calculated binding energy, interfacial energy, Ni–Bi interaction, and mean-square displacement, interfacial energy, a comprehensive demonstration of surface energy and atomic interactions, is proposed as a potential descriptor for predicating and estimating the catalytic performance of the molten alloy-based catalysts.
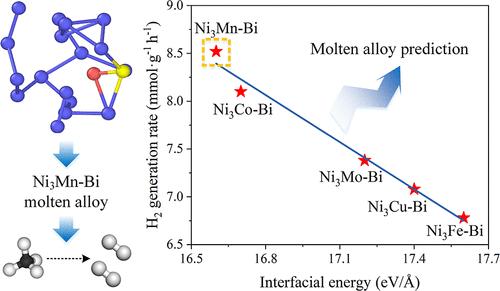
调节Ni-Bi熔融合金界面能促进甲烷分解成氢
催化甲烷分解是一种极具发展前景的无co2制氢技术。然而,开发能够在中等温度下有效分解甲烷的催化剂仍然具有挑战性。在这项研究中,我们开发了一系列具有不同Ni:Mn比的NiMn-Bi熔融合金催化剂,用于催化甲烷分解。mn改性的Ni-Bi合金在850℃时CH4转化率为15.3%,产氢率比Ni-Bi合金提高了112%。该三元合金催化剂在此生产速率下也表现出长达80小时的稳定性。分子动力学模拟表明,Mn的引入显著降低了活性金属Ni与溶剂金属Bi之间的强相互作用,从而加快了甲烷的解离速率。更重要的是,在理论计算的结合能、界面能、Ni-Bi相互作用和均方位移中,界面能作为表面能和原子相互作用的综合表征,被提出作为预测和估计熔融合金基催化剂催化性能的潜在描述符。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Catalysis
CHEMISTRY, PHYSICAL-
CiteScore
20.80
自引率
6.20%
发文量
1253
审稿时长
1.5 months
期刊介绍:
ACS Catalysis is an esteemed journal that publishes original research in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. It offers broad coverage across diverse areas such as life sciences, organometallics and synthesis, photochemistry and electrochemistry, drug discovery and synthesis, materials science, environmental protection, polymer discovery and synthesis, and energy and fuels.
The scope of the journal is to showcase innovative work in various aspects of catalysis. This includes new reactions and novel synthetic approaches utilizing known catalysts, the discovery or modification of new catalysts, elucidation of catalytic mechanisms through cutting-edge investigations, practical enhancements of existing processes, as well as conceptual advances in the field. Contributions to ACS Catalysis can encompass both experimental and theoretical research focused on catalytic molecules, macromolecules, and materials that exhibit catalytic turnover.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


