Continuous Manufacturing of Rocuronium Bromide Part 2: Step 2 Process Development
IF 3.5
3区 化学
Q2 CHEMISTRY, APPLIED
引用次数: 0
Abstract
Rocuronium bromide, a neuromuscular blocking agent critical to anesthesia and emergency care, experienced severe shortages during the COVID-19 pandemic due to fragmented global supply chains and limitations of traditional batch manufacturing. To enhance supply chain resilience and responsiveness, this Part 2 manuscript details the development and optimization of the Step 2 unit operations, including the N-alkylation, continuous reaction, and crystallization. Through kinetic modeling and real-time monitoring using ReactIR and conductivity, the Step 2 N-alkylation was optimized to reduce reaction time and reagent usage. A five-stage CSTR cascade was implemented, achieving >98% rocuronium bromide purity with steady-state performance and minimal residual starting material. For crystallization, extensive solubility and solvent screening identified methyl propyl ketone (MPK) as the most effective antisolvent, enabling high-purity rocuronium bromide recovery with yields up to 94%. The process was successfully scaled to a 500 mL CSTR, and in-line particle analysis confirmed robust crystal growth dynamics. These advancements demonstrate the viability of transitioning small molecule API synthesis into continuous operation while maintaining control over reaction and solid-state quality. The work presented here makes significant progress on the second half of the process (particularly in addressing the low assay challenge), positioning this work for future integration into an end-to-end continuous manufacturing platform for rocuronium bromide.
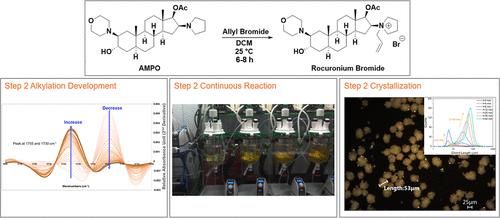
罗库溴铵的连续生产第2部分:步骤2工艺开发
罗库溴铵是一种对麻醉和急救至关重要的神经肌肉阻断剂,在COVID-19大流行期间,由于全球供应链碎片化和传统批量生产的限制,罗库溴铵出现了严重短缺。为了提高供应链的弹性和响应能力,本第2部分手稿详细介绍了步骤2单元操作的开发和优化,包括n -烷基化,连续反应和结晶。通过动力学建模和ReactIR和电导率实时监测,优化了步骤2 n -烷基化反应,减少了反应时间和试剂使用量。采用五级CSTR级联反应,实现了98%的罗库溴铵纯度,具有稳态性能和最小的起始物质残留。对于结晶,广泛的溶解度和溶剂筛选鉴定甲基丙基酮(MPK)是最有效的抗溶剂,使高纯度罗库溴铵的回收率达到94%。该工艺成功地扩展到500 mL CSTR,在线颗粒分析证实了强大的晶体生长动力学。这些进步证明了将小分子原料药合成转变为连续操作的可行性,同时保持对反应和固态质量的控制。本文介绍的工作在该过程的后半部分取得了重大进展(特别是在解决低含量挑战方面),将这项工作定位为未来整合到罗库溴铵的端到端连续制造平台中。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
6.90
自引率
14.70%
发文量
251
审稿时长
2 months
期刊介绍:
The journal Organic Process Research & Development serves as a communication tool between industrial chemists and chemists working in universities and research institutes. As such, it reports original work from the broad field of industrial process chemistry but also presents academic results that are relevant, or potentially relevant, to industrial applications. Process chemistry is the science that enables the safe, environmentally benign and ultimately economical manufacturing of organic compounds that are required in larger amounts to help address the needs of society. Consequently, the Journal encompasses every aspect of organic chemistry, including all aspects of catalysis, synthetic methodology development and synthetic strategy exploration, but also includes aspects from analytical and solid-state chemistry and chemical engineering, such as work-up tools,process safety, or flow-chemistry. The goal of development and optimization of chemical reactions and processes is their transfer to a larger scale; original work describing such studies and the actual implementation on scale is highly relevant to the journal. However, studies on new developments from either industry, research institutes or academia that have not yet been demonstrated on scale, but where an industrial utility can be expected and where the study has addressed important prerequisites for a scale-up and has given confidence into the reliability and practicality of the chemistry, also serve the mission of OPR&D as a communication tool between the different contributors to the field.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


