Industrial Applications of Ionic Liquids in Acetonitrile–Water Azeotrope Separation: Molecular Dynamics and DFT Insights into Azeotrope Breaking Methods
IF 3.9
3区 工程技术
Q2 ENGINEERING, CHEMICAL
引用次数: 0
Abstract
Separating acetonitrile–water azeotropic systems is challenging due to unclear molecular-level interaction mechanisms of ionic liquids (ILs). This study investigated ILs ([Emim]Br, [Bmim]Br, [Emim][BF4], and [Bmim][BF4]) as entrainers using experimental and theoretical approaches to elucidate the azeotropic point-breaking mechanism. Molecular dynamics (MD) simulations were employed to analyze binary and ternary solutions, while density functional theory (DFT) at B3LYP/6–311 + G(d) was used to refine the complexation energies and Boltzmann-averaged multiple isomers. Vapor–liquid equilibrium (VLE) data (T, x, and y) for the pseudobinary systems of acetonitrile, water, and ILs at 101.33 kPa were measured and correlated using the nonrandom two-liquid (NRTL) model. The results show that ILs exhibit a stronger salting-out effect than solvation, enhancing the relative volatility of acetonitrile and eliminating the azeotropic point. The salting-out efficacy followed the order [Emim]Br > [Bmim]Br > [Emim][BF4] > [Bmim][BF4], with [Emim]+ and Br– being the most effective. These findings highlight the ILs’ potential for efficient, sustainable industrial azeotrope separation.
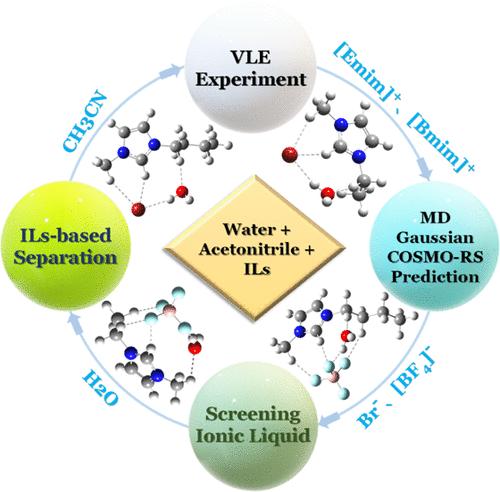
离子液体在乙腈-水共沸分离中的工业应用:分子动力学和DFT对共沸分离方法的见解
由于离子液体分子水平相互作用机制不明确,分离乙腈-水共沸体系具有挑战性。本研究采用实验和理论方法研究了[Emim]Br、[Bmim]Br、[Emim][BF4]和[Bmim][BF4]作为夹带剂的共沸破点机理。采用分子动力学(MD)模拟分析二元和三元解,采用密度泛函理论(DFT)在B3LYP/ 6-311 + G(d)处优化络合能和玻尔兹曼平均多重异构体。使用非随机双液(NRTL)模型测量了101.33 kPa下乙腈、水和il伪二元体系的气液平衡(VLE)数据(T, x和y)并进行了关联。结果表明,溶剂化比溶剂化具有更强的盐析作用,提高了乙腈的相对挥发性,消除了共沸点。盐析效果依次为[Emim]Br >; [Bmim]Br > [Emim][BF4] > [Bmim][BF4],其中[Emim]+和Br -的盐析效果最好。这些发现突出了ILs在高效、可持续的工业共沸物分离方面的潜力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Industrial & Engineering Chemistry Research
工程技术-工程:化工
CiteScore
7.40
自引率
7.10%
发文量
1467
审稿时长
2.8 months
期刊介绍:
ndustrial & Engineering Chemistry, with variations in title and format, has been published since 1909 by the American Chemical Society. Industrial & Engineering Chemistry Research is a weekly publication that reports industrial and academic research in the broad fields of applied chemistry and chemical engineering with special focus on fundamentals, processes, and products.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


