Extensive Evaluation of Dinitropyrazole and Tetrazole-Based Energetic Compounds: Role of Isomerism and Oxygen Balance on the Overall Performance
IF 3.6
2区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
Nitrogen-rich heterocycles have been instrumental in the pursuit of design, synthesis, and modification of advanced energetic materials developed to fulfill evolving requirements. Thus, the role of material chemists is to bridge the research gap by understanding how different rings and functional groups influence overall performance. In this work, 3,4-dinitritropyrazole (3,4-DNP) and 3,5-dinitropyrazole (3,5-DNP) were taken as precursors for the synthesis of energetic materials having harmonized energy and safety. Utilizing N-methylene-C bridging, they were paired with tetrazole and N-hydroxytetrazole explosophores, followed by salt formation for performance modification. The isomeric dinitropyrazoles were found to be more favorable for pairing with the N-hydroxytetrazole than commonly utilized 4-substituted 3,5-dinitropyrazoles. Consequently, this marks the first report of an asymmetric combination involving the N-hydroxytetrazole moiety, achieved through the bridging approach. After the performance evaluation, a comparative assessment of isomeric analogues was implemented. The presence of an additional oxygen supplement results in higher energetic properties with respect to tetrazole-based compounds. All compounds demonstrated energetic performance superior to TNT and good physical stability. This study revealed that among the isomeric 3,4-DNP and 3,5-DNP derivatives, the energetic performance of neutral 3,4-DNP derivatives (7 and 15) was higher, and the thermal stability of the 3,5-DNP derivatives (8 and 16) was superior.
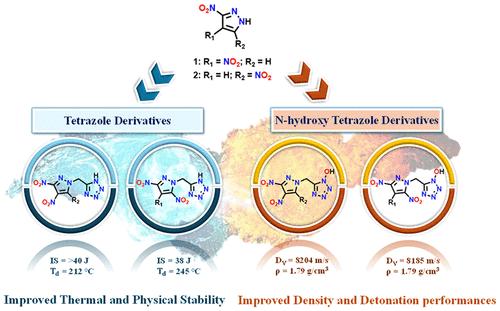
二硝基吡唑和四氮唑类含能化合物的广泛评价:同分异构和氧平衡对整体性能的影响
富氮杂环在追求设计、合成和修饰先进的高能材料以满足不断发展的需求方面发挥了重要作用。因此,材料化学家的作用是通过了解不同的环和官能团如何影响整体性能来弥合研究差距。本文以3,4-二硝基吡唑(3,4- dnp)和3,5-二硝基吡唑(3,5- dnp)为前驱体,合成了能量与安全性一致的含能材料。利用n -亚甲基- c桥接,将它们与四氮唑和n -羟基四氮唑炸药配对,然后形成盐进行性能改性。发现同分异构体二硝基吡唑比常用的4-取代3,5-二硝基吡唑更有利于与n -羟基四唑配对。因此,这标志着通过桥接方法实现的涉及n -羟基四唑部分的不对称组合的第一个报告。性能评估后,对异构体类似物进行了比较评估。额外的氧补充的存在导致相对于四唑基化合物具有更高的能量特性。所有化合物均表现出优于TNT的能量性能和良好的物理稳定性。本研究发现,在3,4- dnp和3,5- dnp异构体衍生物中,中性3,4- dnp衍生物(7和15)的能量性能较高,3,5- dnp衍生物(8和16)的热稳定性较好。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


