Mechanistic Study of Functional Electrolyte Solvents for High-Voltage Lithium Batteries
IF 8.2
2区 材料科学
Q1 MATERIALS SCIENCE, MULTIDISCIPLINARY
引用次数: 0
Abstract
The pervasive use of Ni-rich cathode active materials, e.g., LiNi0.8Mn0.1Co0.1O2 (NMC811), for high-energy-density Li-ion batteries (LIBs) has been hindered by rapid battery capacity decay when cycled with high charge cutoff voltages due to electrolyte decomposition in the conventional carbonate solvent-based electrolytes, oxidative parasitic side reactions at the electrolyte/cathode interface, and irreversible phase changes in the cathode active materials leading to dissolution of transition metals into the electrolytes. Various functional electrolyte solvents have been studied to tackle the above technical challenges, yet the roles of individual solvents in the performance of LIBs remain poorly understood. In this study, we systematically investigate electrochemical performance mechanisms of fluorinated and organosilicon single solvents and cosolvents, for the first time, in high-voltage Li/NMC811 batteries, using electrochemical and analytical characterizations and density functional theory modeling. We observe that some unique combinations of the functional solvents can lead to exceptionally stable high-voltage cycle performance in the Ni-rich cathode-based LIBs. Our mechanistic study reveals that the synergistic effect of solvents plays a vital role in enabling electrochemical stability at both the Ni-rich cathode and the Li metal anode. Understanding the electrochemical performance mechanisms of functional solvents can greatly help in designing and formulating advanced electrolytes that enable the development of high-voltage, high-energy-density, long-cycle-life lithium batteries.
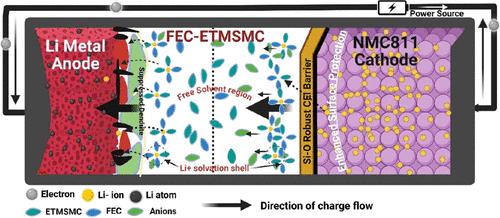
高压锂电池用功能电解质溶剂的机理研究
由于传统碳酸盐溶剂基电解质中的电解质分解、电解质/阴极界面的氧化寄生副反应、电解液/阴极界面的氧化寄生副反应等因素,在高电荷截止电压下循环时,电池容量迅速衰减,阻碍了富镍阴极活性材料(如LiNi0.8Mn0.1Co0.1O2 (NMC811))在高能量密度锂离子电池(LIBs)中的广泛应用。阴极活性材料的不可逆相变导致过渡金属溶解到电解质中。人们已经研究了各种功能电解质溶剂来解决上述技术挑战,但对单个溶剂在lib性能中的作用仍然知之甚少。在这项研究中,我们首次系统地研究了氟化和有机硅单一溶剂和共溶剂在高压Li/NMC811电池中的电化学性能机理,采用电化学和分析表征以及密度泛函理论建模。我们观察到一些独特的功能溶剂组合可以导致富镍阴极基锂离子电池异常稳定的高压循环性能。我们的机理研究表明,溶剂的协同效应在富镍阴极和锂金属阳极的电化学稳定性中起着至关重要的作用。了解功能溶剂的电化学性能机制可以极大地帮助设计和配制先进的电解质,从而开发出高电压、高能量密度、长循环寿命的锂电池。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Applied Materials & Interfaces
工程技术-材料科学:综合
CiteScore
16.00
自引率
6.30%
发文量
4978
审稿时长
1.8 months
期刊介绍:
ACS Applied Materials & Interfaces is a leading interdisciplinary journal that brings together chemists, engineers, physicists, and biologists to explore the development and utilization of newly-discovered materials and interfacial processes for specific applications. Our journal has experienced remarkable growth since its establishment in 2009, both in terms of the number of articles published and the impact of the research showcased. We are proud to foster a truly global community, with the majority of published articles originating from outside the United States, reflecting the rapid growth of applied research worldwide.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


