NH3 annealing for preparing Pt nanocluster@N-doped hollow mesoporous carbon spheres with enhanced hydroxyl aldehyde hydrogenation
IF 4.3
2区 工程技术
Q2 ENGINEERING, CHEMICAL
引用次数: 0
Abstract
The development of nitrogen-doped mesoporous carbon spheres (N-CS) integrated with metal nanoclusters for enhanced hydroxyl aldehyde hydrogenation remains challenging. In this work, a NH3 annealing strategy was employed to prepare Pt nanocluster-loaded nitrogen-doped mesoporous carbon spheres (Pt@N-CS) catalysts. In Pt@N-CS, Pt nanoclusters are anchored onto nitrogen-doped carbon spheres via strong Pt–N interactions, which facilitate electron transfer and effectively suppress Pt leaching and agglomeration. The hydrogenation of 2-ethyl-3-hydroxyhexanal using Pt@N-CS shows nearly 98 % conversion and yield, with selectivity close to 100 % under optimal conditions. Density functional theory calculations reveal that nitrogen doping induces electron-deficient Pt sites, which enhance the adsorption and activation of H2 (−0.93 eV) and EHA (−1.66 eV). The charge redistribution at the metal-support interface could strengthen the interaction between Pt and the carbonyl group of 2-ethyl-3-hydroxyhexanal. Consequently, the energy barrier of the rate-determining step is reduced by 0.06 eV, thereby facilitating the hydrogenation process under mild conditions.
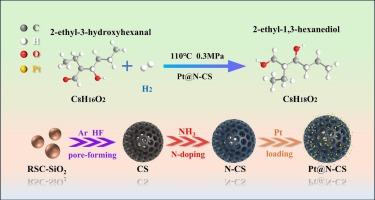
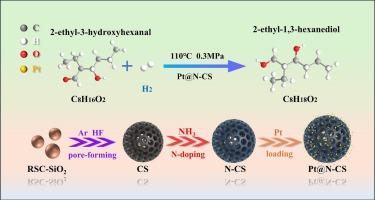
NH3退火制备Pt nanocluster@N-doped羟基醛加氢中空介孔碳球
结合金属纳米团簇的氮掺杂介孔碳球(N-CS)增强羟基醛加氢的研究仍然具有挑战性。在这项工作中,采用NH3退火策略制备了Pt纳米团簇负载氮掺杂介孔碳球(Pt@N-CS)催化剂。在Pt@N-CS中,Pt纳米团簇通过强Pt - n相互作用锚定在氮掺杂碳球上,促进电子转移,有效抑制Pt浸出和团聚。Pt@N-CS加氢2-乙基-3-羟基己醛的转化率和产率接近98 %,在最佳条件下选择性接近100% %。密度泛函理论计算表明,氮掺杂诱导了缺电子Pt位,增强了H2(- 0.93 eV)和EHA(- 1.66 eV)的吸附和活化。金属-载体界面的电荷重分配增强了Pt与2-乙基-3-羟基己醛羰基之间的相互作用。因此,决定速率步骤的能垒降低了0.06 eV,从而有利于温和条件下的加氢过程
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Chemical Engineering Science
工程技术-工程:化工
CiteScore
7.50
自引率
8.50%
发文量
1025
审稿时长
50 days
期刊介绍:
Chemical engineering enables the transformation of natural resources and energy into useful products for society. It draws on and applies natural sciences, mathematics and economics, and has developed fundamental engineering science that underpins the discipline.
Chemical Engineering Science (CES) has been publishing papers on the fundamentals of chemical engineering since 1951. CES is the platform where the most significant advances in the discipline have ever since been published. Chemical Engineering Science has accompanied and sustained chemical engineering through its development into the vibrant and broad scientific discipline it is today.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


