Engineered tumor-symbiotic bacterial membrane nanovesicles enable precise immuno-chemotherapy of colorectal cancer
IF 11.5
1区 医学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Gut microorganisms show promising therapeutic effects and drug delivery potential for colorectal cancer (CRC) treatment, but are limited by their insufficient targeting ability and side effects. Fusobacterium nucleatum (Fn) is a key symbiotic bacterium in CRC, which can preferentially accumulate in tumor tissues and invade tumor cells, while its tumorigenicity restricts the application in drug delivery. Herein, we engineered Fn with anchored PD-L1 antibody (αPD-L1), and then isolated the Fn membranes to construct bacterial membrane nanovesicles (ab-FMNVs) for precise delivery of chemotherapeutic drugs. The ab-FMNVs exploited Fn's inherent tumor colonization capabilities to achieve tumor-targeted delivery through the specific membrane protein FadA-mediated pathway, and modulated the PD-L1 immune checkpoint pathway for tumor immunotherapy. Simultaneously, ab-FMNVs were internalized into CT26 cells to release the chemotherapeutic agent doxorubicin, synergistically inhibiting tumor cell proliferation and metastasis. In a CRC-bearing mouse model, doxorubicin-loaded ab-FMNVs increased tumor accumulation and demonstrated superior antitumor efficacy against both primary and recurrent CRC progression without inducing any side effects. This innovative approach holds promise for precision cancer therapies by harnessing the symbiotic relationship between bacteria and CRC.
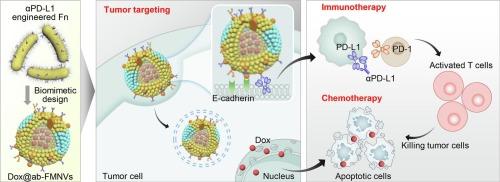
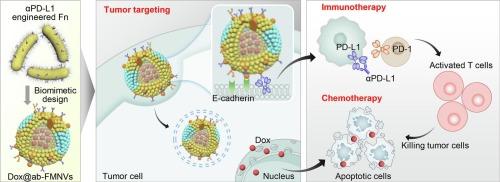
工程肿瘤共生细菌膜纳米囊泡使结直肠癌的精确免疫化疗成为可能
肠道微生物在结直肠癌(CRC)治疗中显示出良好的治疗效果和药物传递潜力,但受其靶向能力不足和副作用的限制。核梭杆菌(Fusobacterium nucleatum, Fn)是结直肠癌的关键共生细菌,可优先在肿瘤组织中积累并侵袭肿瘤细胞,但其致瘤性限制了其在给药领域的应用。在本研究中,我们用锚定的PD-L1抗体(αPD-L1)修饰Fn,然后分离Fn膜,构建细菌膜纳米囊泡(ab-FMNVs),用于精确递送化疗药物。抗体- fmnv利用Fn固有的肿瘤定植能力,通过特异性膜蛋白fada介导的途径实现肿瘤靶向递送,并调节PD-L1免疫检查点途径,用于肿瘤免疫治疗。同时,将ab- fmnv内化到CT26细胞中释放化疗药物阿霉素,协同抑制肿瘤细胞的增殖和转移。在携带CRC的小鼠模型中,负载阿霉素的ab-FMNVs增加了肿瘤积累,并对原发性和复发性CRC进展表现出卓越的抗肿瘤功效,且没有引起任何副作用。通过利用细菌和结直肠癌之间的共生关系,这种创新的方法有望实现精确的癌症治疗。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Controlled Release
医学-化学综合
CiteScore
18.50
自引率
5.60%
发文量
700
审稿时长
39 days
期刊介绍:
The Journal of Controlled Release (JCR) proudly serves as the Official Journal of the Controlled Release Society and the Japan Society of Drug Delivery System.
Dedicated to the broad field of delivery science and technology, JCR publishes high-quality research articles covering drug delivery systems and all facets of formulations. This includes the physicochemical and biological properties of drugs, design and characterization of dosage forms, release mechanisms, in vivo testing, and formulation research and development across pharmaceutical, diagnostic, agricultural, environmental, cosmetic, and food industries.
Priority is given to manuscripts that contribute to the fundamental understanding of principles or demonstrate the advantages of novel technologies in terms of safety and efficacy over current clinical standards. JCR strives to be a leading platform for advancements in delivery science and technology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


