Al Pinning Effect in Birnessite for High-Performance Ammonium-Ion Storage
IF 26.8
1区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Layered birnessite has attracted considerable attention for its cathode potential in various aqueous energy storage devices owing to its two-electron transfer reaction (Mn2+/Mn4+), open diffusion channels, and tunable interlayer spacings. However, birnessite for reversible ammonium (NH4+) ion storage generally suffers from irreversible structural collapse originated from Jahn–Teller (J–T) effect of Mn3+ and the intrinsic slow ionic diffusion kinetics. Herein, an Al pinning effect in birnessite is found to address these two issues simultaneously, which promoted enhanced structural stability and resulted in fast ionic diffusion kinetics for excellent high-rate capability. Strikingly, a robust cycling stability over 5, 000 cycles at 1.0 A g−1 is achieved in the optimal Na0.7Al0.1Mn0.9O2, which surpasses that of most previously reported ammonium-ion batteries. Density functional theory calculations revealed that the pinned [Al3+O6] octahedra not only decrease the Mn3+ content in birnessite, but also strengthen the covalency of Mn─O bonds to resist the collinear elongation/compression direction of the [Mn3+O6] octahedra. Furthermore, Al pinning in birnessite can increase the interlayer spacing due to the regulation of Mn3+─O/Mn4+─O bond length and decrease the diffusion barrier for NH4+ ion in the interlayer of birnessite. Thus, an accelerated NH4+ ion diffusion coefficient of 1.58 × 10−9 cm2 s−1 has been achieved, which is ≈5 times higher than of the pristine one and also higher than that in other cathode materials. The findings demonstrate that layered Na0.7Al0.1Mn0.9O2 is a very promising cathode candidate for NH4+ ion battery, and the Al pinning effect in birnessite can effectively suppress the J–T effect and enhance the NH4+ ion diffusion kinetics simultaneously.
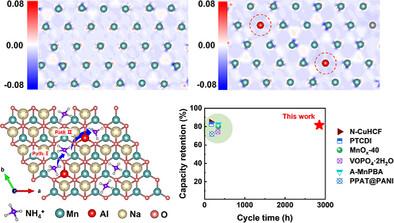
铝钉钉效应在硼钛矿中用于高性能铵离子存储
层状碳化硅由于其双电子转移反应(Mn2+/Mn4+)、开放的扩散通道和可调的层间间距,在各种水储能装置中具有阴极电位,引起了人们的广泛关注。然而,用于可逆铵离子存储的birnite通常存在由Mn3+的Jahn-Teller (J-T)效应引起的不可逆结构坍塌和固有的缓慢离子扩散动力学。本文发现,铝钉钉效应同时解决了这两个问题,提高了结构稳定性,并产生了快速的离子扩散动力学,从而获得了优异的高速率性能。引人注目的是,在最佳的Na0.7Al0.1Mn0.9O2中,在1.0 a g−1下实现了超过5000次循环的强大循环稳定性,这超过了之前报道的大多数铵离子电池。密度泛函理论计算表明,钉住的[Al3+O6]八面体不仅降低了碳化硅中Mn3+的含量,而且增强了Mn─O键的共价,抵抗了[Mn3+O6]八面体的共线伸长/压缩方向。此外,铝钉钉在铌铁矿中的作用通过调节Mn3+─O/Mn4+─O键长,增加了铌铁矿层间间距,降低了NH4+离子在铌铁矿层间的扩散屏障。因此,NH4+离子的加速扩散系数达到了1.58 × 10−9 cm2 s−1,是原始材料的约5倍,也高于其他正极材料。研究结果表明,层状Na0.7Al0.1Mn0.9O2是一种极有前途的NH4+离子电池阴极候选材料,铝钉钉效应可以有效抑制J-T效应,同时增强NH4+离子的扩散动力学。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Advanced Materials
工程技术-材料科学:综合
CiteScore
43.00
自引率
4.10%
发文量
2182
审稿时长
2 months
期刊介绍:
Advanced Materials, one of the world's most prestigious journals and the foundation of the Advanced portfolio, is the home of choice for best-in-class materials science for more than 30 years. Following this fast-growing and interdisciplinary field, we are considering and publishing the most important discoveries on any and all materials from materials scientists, chemists, physicists, engineers as well as health and life scientists and bringing you the latest results and trends in modern materials-related research every week.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


