Embedded Fe-Cu Pairs Enable Tandem Nitrate-to-Ammonia Electroreduction
IF 26.8
1区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
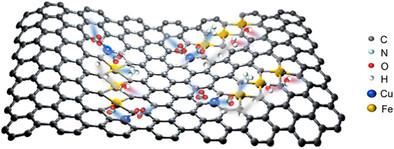
Electrochemical nitrate reduction (e-NO3RR) to ammonia (NH3) represents a transformative technology that seamlessly integrates environmental remediation with resource regeneration. This approach is crucial for restoring equilibrium in the global nitrogen cycling, advancing green chemistry, and accelerating the transition toward a sustainable circular economy. However, under pH-neutral conditions, the simultaneous occurrence of two competing reactions (Hydrogen Evolution Reaction and NO3RR) at the same active sites results in considerable interference, significantly limiting the catalytic efficiency and selectivity. Here a Fe-Cu pair (Cu-N3/Fe3-N8) electrocatalyst is meticulously designed, achieving a NH3 production rate of 18.83 mg∙h‒1∙mgcat‒1 at −0.65 V versus the reversible hydrogen electrode (RHE), accompanied with a Faradaic efficiency of 97.1%. This as-prepared Fe-Cu pair overcomes the limitations of conventional bimetallic catalysts, which typically rely on direct atomic coupling. The electron-deficient region formed by Cu–N3 enhances the adsorption of nitrate, while the electron-rich domain generated by the Fe3–N8 cluster facilitates the adsorption of nitrite and promotes water activation. The spatially separated charge gradient optimizes the adsorption energies of multi-step reaction intermediates, thereby establishing a relay mechanism. The work provides valuable insights into the design of multi-active-site electrocatalysts and offers a promising approach to addressing critical challenges in nitrogen resource conversion.
嵌入式Fe-Cu对使串联硝酸盐到氨电还原
电化学硝酸还原(e-NO3RR)制氨(NH3)是一项将环境修复与资源再生无缝结合的变革性技术。这种方法对于恢复全球氮循环平衡、推进绿色化学和加速向可持续循环经济过渡至关重要。但在ph中性条件下,在同一活性位点同时发生析氢反应和NO3RR两种相互竞争的反应会产生相当大的干扰,极大地限制了催化效率和选择性。本文精心设计了Fe-Cu对(Cu-N3/Fe3-N8)电催化剂,与可逆氢电极(RHE)相比,在−0.65 V下NH3的产率为18.83 mg∙h-1∙mgcat-1,法拉第效率为97.1%。这种制备的Fe-Cu对克服了传统双金属催化剂通常依赖于直接原子偶联的局限性。Cu-N3形成的缺电子区增强了对硝酸盐的吸附,而Fe3-N8簇形成的富电子区有利于亚硝酸盐的吸附,促进了水的活化。空间分离的电荷梯度优化了多步反应中间体的吸附能,从而建立了一种接力机制。这项工作为多活性位点电催化剂的设计提供了有价值的见解,并为解决氮资源转化中的关键挑战提供了有前途的方法。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Advanced Materials
工程技术-材料科学:综合
CiteScore
43.00
自引率
4.10%
发文量
2182
审稿时长
2 months
期刊介绍:
Advanced Materials, one of the world's most prestigious journals and the foundation of the Advanced portfolio, is the home of choice for best-in-class materials science for more than 30 years. Following this fast-growing and interdisciplinary field, we are considering and publishing the most important discoveries on any and all materials from materials scientists, chemists, physicists, engineers as well as health and life scientists and bringing you the latest results and trends in modern materials-related research every week.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


