Highly Selective and Near-Complete Electrochemical Recovery of Cobalt and Nickel from Spent Batteries through Mutifunctional Deep Eutectic Solvent
IF 20.2
1区 材料科学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
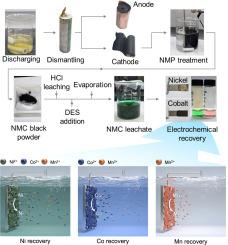
Electrochemical recovery presents a sustainable route for battery recycling, yet it is hindered by a trade-off between achieving purity and yield. This challenge arises because, as the target metal depletes during electrodeposition, mass transport limitations reduce its availability, thereby shifting the electrochemical environment in favor of co-deposition of competing metal – particularly during prolonged deposition intended for near-complete recovery. Here, we report a strategy that leverages a multifunctional deep eutectic solvent (DES), ethaline, where ethylene glycol preferentially coordinates with nickel while chloride stabilizes cobalt as tetrachlorocobaltate complexes. Even at elevated temperatures, where nickel undergoes a partial thermochromic transition to chloride coordination, the system maintains a broadened Ni–Co separation window of ∼0.3 V at 85°C. By fine-tuning the applied potential and utilizing the intrinsic chlorine redox activity of the DES, self-purification was achieved during electrodeposition, yielding a Ni/Co separation factor >3,000 and >97% nickel recovery in a single-step electrodeposition from a synthetic Ni/Co mixture. Building upon this binary separation, we developed a sequential strategy to recover nickel, cobalt, and manganese from real battery leachates. Applied to real NMC leachates, our process enabled the sequential recovery of nickel, cobalt, and manganese with purities of 99.1%/96.3% (NMC111) and 99.2%/98.8% (NMC811) for nickel and cobalt, respectively, all with >95% recovery. For NMC111, >97% nickel purity and >93% cobalt purity were retained over repeated reuse of the DES, enabling minimal wastewater discharge, with Cl2-assisted refining enhancing purity to >99.9%. A technoeconomic analysis validated the economic feasibility and revealed further potential through thermal optimization.
利用多功能深共晶溶剂从废电池中高选择性、近完全电化学回收钴和镍
电化学回收为电池回收提供了一条可持续的途径,但在实现纯度和产量之间的权衡阻碍了这一点。这一挑战的出现是因为,随着目标金属在电沉积过程中耗尽,质量传输限制降低了其可用性,从而改变了电化学环境,有利于竞争金属的共沉积,特别是在为接近完全回收而延长沉积时间的过程中。在这里,我们报告了一种利用多功能深共晶溶剂(DES)的策略,乙炔,乙二醇优先与镍配合,而氯化物稳定钴为四氯钴酸盐配合物。即使在高温下,镍经历了部分热致变色转变为氯化物配位,该系统在85°C时保持了宽的Ni-Co分离窗口~ 0.3 V。通过微调应用电位和利用DES的固有氯氧化还原活性,电沉积过程中实现了自净化,从合成的Ni/Co混合物中获得了Ni/Co分离系数>; 3000和>;97%的镍回收率。在这种二元分离的基础上,我们开发了一种从真正的电池渗滤液中回收镍、钴和锰的顺序策略。应用于实际的NMC渗滤液中,我们的工艺能够依次回收镍、钴和锰,镍和钴的纯度分别为99.1%/96.3% (NMC111)和99.2%/98.8% (NMC811),回收率均为95%。对于NMC111,在重复使用DES的过程中,镍纯度保持在97%,钴纯度保持在93%,废水排放量最小,cl2辅助精炼将纯度提高到99.9%。技术经济分析验证了经济可行性,并通过热优化揭示了进一步的潜力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Energy Storage Materials
Materials Science-General Materials Science
CiteScore
33.00
自引率
5.90%
发文量
652
审稿时长
27 days
期刊介绍:
Energy Storage Materials is a global interdisciplinary journal dedicated to sharing scientific and technological advancements in materials and devices for advanced energy storage and related energy conversion, such as in metal-O2 batteries. The journal features comprehensive research articles, including full papers and short communications, as well as authoritative feature articles and reviews by leading experts in the field.
Energy Storage Materials covers a wide range of topics, including the synthesis, fabrication, structure, properties, performance, and technological applications of energy storage materials. Additionally, the journal explores strategies, policies, and developments in the field of energy storage materials and devices for sustainable energy.
Published papers are selected based on their scientific and technological significance, their ability to provide valuable new knowledge, and their relevance to the international research community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


