Injectable smart hydrogel with dual pH/thermal responsiveness: A PCL–PEG–PCL/niosome synergistic platform for precision drug delivery
IF 6
2区 医学
Q2 MATERIALS SCIENCE, BIOMATERIALS
Materials Science & Engineering C-Materials for Biological Applications
Pub Date : 2025-09-25
DOI:10.1016/j.bioadv.2025.214527
引用次数: 0
Abstract
Achieving site-specific, on-demand drug release in response to physiological stimuli remains a critical hurdle in precision medicine. In this study, we introduce a smart, injectable nanocomposite hydrogel that combines thermoresponsive behavior with a degradation-triggered pH-responsive mechanism to enable precise and controlled therapeutic delivery. This platform is based on poly(ε-caprolactone)-b-poly(ethyleneglycol)-b-poly(ε-caprolactone) (PCEC) hydrogel matrix, co-loaded with dexamethasone (DEX) and ZnO nanoparticles encapsulated in niosomes (N/DEX/ZnO). The PCEC hydrogel, synthesized through ring-opening polymerization, exhibited thermoresponsive in situ gelation at physiological temperature, enabling formation of a stable depot at the injection site. Critically, the slow degradation of the hydrophobic, semicrystalline PCEC matrix (∼37 % over one month) produced a localized drop in pH (from 7.4 to ∼5.5), shifting the microenvironment from neutral (pH 7.4) to acidic (approximately pH 5.5). This localized acidification triggered ZnO nanoparticle dissolution, which in turn enabled a controlled, pH-sensitive release of drug from the niosomal carriers. In vitro release studies demonstrated a significantly enhanced cumulative release of DEX under acidic conditions, characterized by a biphasic release profile. Cell viability assays using human foreskin fibroblast (HFF) cells confirmed excellent cytocompatibility, with cell viability exceeding 85 % after 24 h of exposure. Additionally, in vivo subcutaneous administration of both PCEC and N/DEX/ZnO@PCEC hydrogels resulted in robust gel formation and favorable histopathological outcomes, with no significant inflammatory responses detected. Collectively, these findings highlight this smart, injectable hydrogel platform as a promising candidate for localized, sustained, and feedback-responsive drug delivery in therapeutic applications.
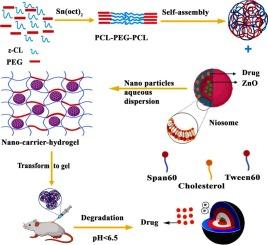
具有双重pH/热响应性的可注射智能水凝胶:用于精确给药的PCL-PEG-PCL/niosome协同平台。
在生理刺激下实现位点特异性、按需药物释放仍然是精准医学的一个关键障碍。在这项研究中,我们介绍了一种智能的、可注射的纳米复合水凝胶,它结合了热响应行为和降解触发的ph响应机制,以实现精确和可控的治疗递送。该平台基于聚(ε-己内酯)-b-聚乙二醇-b-聚(ε-己内酯)(PCEC)水凝胶基质,共负载包裹在纳米体(N/DEX/ZnO)中的地塞米松(DEX)和ZnO纳米颗粒。通过开环聚合合成的PCEC水凝胶,在生理温度下表现出热响应性原位凝胶,能够在注射部位形成稳定的储库。关键是,疏水半结晶PCEC基质的缓慢降解(在一个月内降解约37%)产生了局部pH下降(从7.4降至5.5),将微环境从中性(pH 7.4)转变为酸性(约pH 5.5)。这种局部酸化触发氧化锌纳米颗粒溶解,从而使药物从离子体载体中受控地、ph敏感地释放出来。体外释放研究表明,在酸性条件下,DEX的累积释放显著增强,具有双相释放特征。使用人包皮成纤维细胞(HFF)细胞进行细胞活力测定证实了良好的细胞相容性,暴露24小时后细胞活力超过85%。此外,体内皮下给药PCEC和N/DEX/ZnO@PCEC水凝胶可形成强大的凝胶和良好的组织病理学结果,未检测到明显的炎症反应。总的来说,这些发现突出了这种智能的、可注射的水凝胶平台作为治疗应用中局部、持续和反馈反应性药物递送的有希望的候选者。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
17.80
自引率
0.00%
发文量
501
审稿时长
27 days
期刊介绍:
Biomaterials Advances, previously known as Materials Science and Engineering: C-Materials for Biological Applications (P-ISSN: 0928-4931, E-ISSN: 1873-0191). Includes topics at the interface of the biomedical sciences and materials engineering. These topics include:
• Bioinspired and biomimetic materials for medical applications
• Materials of biological origin for medical applications
• Materials for "active" medical applications
• Self-assembling and self-healing materials for medical applications
• "Smart" (i.e., stimulus-response) materials for medical applications
• Ceramic, metallic, polymeric, and composite materials for medical applications
• Materials for in vivo sensing
• Materials for in vivo imaging
• Materials for delivery of pharmacologic agents and vaccines
• Novel approaches for characterizing and modeling materials for medical applications
Manuscripts on biological topics without a materials science component, or manuscripts on materials science without biological applications, will not be considered for publication in Materials Science and Engineering C. New submissions are first assessed for language, scope and originality (plagiarism check) and can be desk rejected before review if they need English language improvements, are out of scope or present excessive duplication with published sources.
Biomaterials Advances sits within Elsevier''s biomaterials science portfolio alongside Biomaterials, Materials Today Bio and Biomaterials and Biosystems. As part of the broader Materials Today family, Biomaterials Advances offers authors rigorous peer review, rapid decisions, and high visibility. We look forward to receiving your submissions!

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


