Electron beam-persulfate system effectively reduces polycyclic aromatic hydrocarbons and Cr(VI) emissions: Environmental matrix impact and mechanism analysis
IF 4.4
3区 环境科学与生态学
Q2 ENVIRONMENTAL SCIENCES
引用次数: 0
Abstract
Heavy metals (HMs) and polycyclic aromatic hydrocarbons (PAHs) are prevalent pollutants in the environment, and their intricate interactions amplify the challenges of remediating complex contaminations. There is an urgent need for effective methods to treat these composite pollutants. This study innovatively demonstrates that the electron beam-persulfate system can effectively remove PAHs and reduce heavy metals. Compared to the sole use of electron beam irradiation, the degradation rate of naphthalene in the electron beam-persulfate system increased by 2.5 times, and the reduction efficiency for hexavalent chromium reached 97.6 %. In comparison with persulfate alone, the treatment efficiency of the electron beam-persulfate system for NAP increased by 8 times, achieving complete degradation at 10 kGy, with the degradation process conforming to pseudo-first-order kinetics. The experimental results indicate that the electron beam-persulfate system is a stable operational system, with pH, liquid depth, types and concentrations of inorganic ions exerting a minor influence on the system. Experimental analysis confirmed that hydroxyl and sulfate radicals play vital roles in PAH removal, while hydrated electrons and sulfate radicals are crucial for the reduction of heavy metals. Toxicity analysis also revealed that the electron beam-persulfate system achieves harmless treatment of complex pollutants. Therefore, the electron beam-persulfate system offers an efficient technology that maintains stability in various environments, providing novel pathways and methods for pollutant removal.
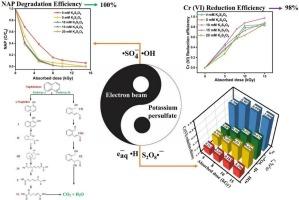
电子束-过硫酸盐体系有效降低多环芳烃和Cr(VI)排放:环境基质影响及机理分析
重金属(HMs)和多环芳烃(PAHs)是环境中普遍存在的污染物,它们之间复杂的相互作用加大了修复复杂污染物的挑战。迫切需要有效的方法来处理这些复合污染物。本研究创新性地证明了电子束-过硫酸盐体系能够有效去除多环芳烃,降低重金属含量。与单纯使用电子束辐照相比,电子束-过硫酸盐体系对萘的降解率提高了2.5倍,对六价铬的还原效率达到97.6%。与单独使用过硫酸盐相比,电子束-过硫酸盐体系对NAP的处理效率提高了8倍,在10 kGy下实现了完全降解,降解过程符合准一级动力学。实验结果表明,电子束-过硫酸盐体系是一个稳定的运行体系,pH、液深、无机离子种类和浓度对体系影响较小。实验分析证实羟基和硫酸盐自由基在PAH的去除中起着至关重要的作用,而水合电子和硫酸盐自由基对重金属的还原至关重要。毒性分析还表明,电子束-过硫酸盐体系对复杂污染物实现了无害化处理。因此,电子束-过硫酸盐系统提供了一种在各种环境中保持稳定性的高效技术,为污染物去除提供了新的途径和方法。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of contaminant hydrology
环境科学-地球科学综合
CiteScore
6.80
自引率
2.80%
发文量
129
审稿时长
68 days
期刊介绍:
The Journal of Contaminant Hydrology is an international journal publishing scientific articles pertaining to the contamination of subsurface water resources. Emphasis is placed on investigations of the physical, chemical, and biological processes influencing the behavior and fate of organic and inorganic contaminants in the unsaturated (vadose) and saturated (groundwater) zones, as well as at groundwater-surface water interfaces. The ecological impacts of contaminants transported both from and to aquifers are of interest. Articles on contamination of surface water only, without a link to groundwater, are out of the scope. Broad latitude is allowed in identifying contaminants of interest, and include legacy and emerging pollutants, nutrients, nanoparticles, pathogenic microorganisms (e.g., bacteria, viruses, protozoa), microplastics, and various constituents associated with energy production (e.g., methane, carbon dioxide, hydrogen sulfide).
The journal''s scope embraces a wide range of topics including: experimental investigations of contaminant sorption, diffusion, transformation, volatilization and transport in the surface and subsurface; characterization of soil and aquifer properties only as they influence contaminant behavior; development and testing of mathematical models of contaminant behaviour; innovative techniques for restoration of contaminated sites; development of new tools or techniques for monitoring the extent of soil and groundwater contamination; transformation of contaminants in the hyporheic zone; effects of contaminants traversing the hyporheic zone on surface water and groundwater ecosystems; subsurface carbon sequestration and/or turnover; and migration of fluids associated with energy production into groundwater.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


