Evaluation of drug distributions in brain tumor therapy: An in silico comparative study of multi-side-hole and standard end-hole catheters in convection-enhanced delivery
IF 5.2
2区 医学
Q1 PHARMACOLOGY & PHARMACY
引用次数: 0
Abstract
Convection-enhanced delivery (CED) is a promising technique for treating brain tumors by enabling drugs to bypass the blood–brain barrier (BBB). The types of catheters and physicochemical properties of drugs both influence drug distribution within tumors. In this study, based on a 3D brain model reconstructed from magnetic resonance (MR) images, a Computational Fluid Dynamics (CFD) simulation is employed to investigate the effects of a multi-side-hole catheter (MSHC) on the CED administration of six anticancer drugs. Results indicate that reducing catheter diameter at the same infusion rate increases the effective volumes of all six drugs. When administered through the MSHC, five of the six drugs (excluding paclitaxel) show increased effective distribution volumes, while none of the drugs demonstrate higher volume-averaged concentrations. In summary, compared to the standard end-hole catheter (SEHC), the MSHC shows superior therapeutic efficacy for doxorubicin, cisplatin, fluorouracil, and carmustine. When methotrexate is administered via MSHC, it is necessary to balance the increased distribution volume against the reduced volume-averaged concentration. Paclitaxel is more suitable for administration in the SEHC, especially with a low infusion rate and a small-diameter catheter. These findings can provide recommendations for the clinical implementation of CED.
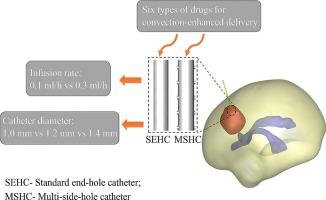
脑肿瘤治疗中药物分布的评价:多侧孔和标准端孔导管对流增强输送的计算机对比研究。
对流增强输送(CED)是一种很有前途的治疗脑肿瘤的技术,它使药物能够绕过血脑屏障(BBB)。导管的类型和药物的理化性质都会影响药物在肿瘤内的分布。在本研究中,基于磁共振(MR)图像重建的三维脑模型,采用计算流体动力学(CFD)模拟研究了多侧孔导管(MSHC)对6种抗癌药物的CED给药的影响。结果表明,在相同输注速率下,减小导管直径可使6种药物的有效容积增加。当通过MSHC给药时,六种药物中的五种(不包括紫杉醇)显示有效分布体积增加,而没有一种药物显示更高的体积平均浓度。综上所述,与标准端孔导管(SEHC)相比,MSHC对阿霉素、顺铂、氟尿嘧啶和卡莫司定的治疗效果更佳。当甲氨蝶呤通过MSHC给药时,有必要平衡增加的分布体积和减少的体积平均浓度。紫杉醇更适合于SEHC的给药,特别是在低输注速率和小直径导管的情况下。这些发现可以为临床实施CED提供建议。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
10.70
自引率
8.60%
发文量
951
审稿时长
72 days
期刊介绍:
The International Journal of Pharmaceutics is the third most cited journal in the "Pharmacy & Pharmacology" category out of 366 journals, being the true home for pharmaceutical scientists concerned with the physical, chemical and biological properties of devices and delivery systems for drugs, vaccines and biologicals, including their design, manufacture and evaluation. This includes evaluation of the properties of drugs, excipients such as surfactants and polymers and novel materials. The journal has special sections on pharmaceutical nanotechnology and personalized medicines, and publishes research papers, reviews, commentaries and letters to the editor as well as special issues.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


