High dose dry powder inhalation of itraconazole nanocrystals: Impact of drug load and inhalation device
IF 4.7
3区 医学
Q1 PHARMACOLOGY & PHARMACY
引用次数: 0
Abstract
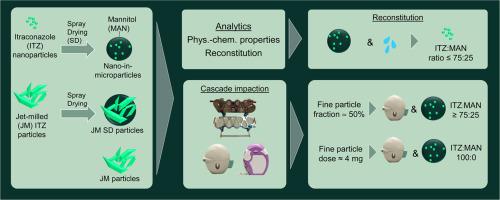
The aerosol performance of dry powders for inhalation depends on the interplay between formulation, device, primary packaging, and patient inhalation profile. This study evaluated a particle engineered formulation for high dose delivery of itraconazole (ITZ), manufactured via wet media milling and consecutive spray drying with mannitol (MAN) as a matrix former.
Key objectives included preserving ITZ nanocrystals and assessing the effects of drug load and inhaler type (HandiHaler® vs. GyroHaler®) on aerosol performance using cascade impaction after automated filling with a drum filler.
Drum filling was suitable for media-milled and spray-dried particles (nano-in-microparticles) but not for jet-milled (JM) or jet-milled and spray-dried (JM SD) samples due to their cohesiveness. Samples JM and JM SD showed significantly lower fine particle fractions (FPF ≤ 26 %) compared to engineered formulations (FPF up to 49 % with the HandiHaler®). The ITZ:MAN ratio did not affect the FPFs of the nano-in-microparticles therefore the fine particle dose (FPD) was primarily determined by the drug loading. The GyroHaler® consistently yielded lower FPFs. The highest FPD of up to 4 mg and highest FPF were achieved with a formulation without MAN using the HandiHaler®, likely due to optimized particle morphology (e.g., low density, smooth surface). Drug loading had no relevant impact on aerosolization as other factors such as particle morphology, surface roughness and the dispersion force of the device were supposed to have the major effect. Nevertheless, higher MAN content improved nanoparticle redispersion: the higher the MAN content, the faster the reconstitution. An ITZ:MAN ratio of 75:25 (resulting in a drug loading of 65 % (w/w) ITZ) was identified as superior formulation with an optimal balance between a high FPD and nearly complete reconstitution of the nanoparticles.
In conclusion, media milling in combination with consecutive spray drying is a suitable formulation technology for high dose dry powder aerosol therapy. Poorly water-soluble compounds with challenging aerosolization parameters could be transformed into particles with much improved aerosol properties superior to those using a conventional jet-milling process. MAN as matrix former increased the redispersibility of the nanoparticles incorporated. The choice of an inhalation device was found crucial for an optimal aerosol performance irrespectively of the dry powder formulation.
伊曲康唑纳米晶体大剂量干粉吸入:药量和吸入装置的影响。
用于吸入的干粉的气溶胶性能取决于配方、设备、主要包装和患者吸入剖面之间的相互作用。本研究评估了一种用于大剂量伊曲康唑(ITZ)的颗粒工程配方,该配方以甘露醇(MAN)为基质成型剂,通过湿介质研磨和连续喷雾干燥制造。主要目标包括保存ITZ纳米晶体和评估药物负荷和吸入器类型(handdihaler®vs. GyroHaler®)在卷筒填料自动填充后使用级联撞击对气溶胶性能的影响。由于介质研磨和喷雾干燥的颗粒(纳米颗粒)具有黏性,因此不适合喷射研磨(JM)或喷射研磨和喷雾干燥(JM SD)样品。与工程配方(handdihaler®的FPF高达49%)相比,样品JM和JM SD的细颗粒分数(FPF≤26%)显着降低。ITZ:MAN比不影响纳米微颗粒的FPD,因此细颗粒剂量主要由载药量决定。GyroHaler®始终产生较低的fpf。在使用handdihaler®的配方中,最高的FPD高达4 mg,最高的FPF可能是由于优化的颗粒形态(例如,低密度,表面光滑)。载药量对雾化效果没有影响,主要影响因素是颗粒形态、表面粗糙度和装置的分散力。然而,更高的MAN含量改善了纳米颗粒的再分散:MAN含量越高,重构越快。经鉴定,在高FPD和纳米颗粒几乎完全重构之间达到最佳平衡的最佳配方为75:25(导致载药量为65% (w/w) ITZ)。综上所述,介质碾磨与连续喷雾干燥相结合是一种适合大剂量干粉气溶胶治疗的配方技术。具有挑战性雾化参数的水溶性较差的化合物可以转化为颗粒,其气溶胶特性比使用传统射流铣削工艺的颗粒要好得多。MAN作为基质成形剂提高了所掺入纳米颗粒的再分散性。吸入装置的选择是至关重要的,无论干粉制剂的最佳气溶胶性能。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
9.60
自引率
2.20%
发文量
248
审稿时长
50 days
期刊介绍:
The journal publishes research articles, review articles and scientific commentaries on all aspects of the pharmaceutical sciences with emphasis on conceptual novelty and scientific quality. The Editors welcome articles in this multidisciplinary field, with a focus on topics relevant for drug discovery and development.
More specifically, the Journal publishes reports on medicinal chemistry, pharmacology, drug absorption and metabolism, pharmacokinetics and pharmacodynamics, pharmaceutical and biomedical analysis, drug delivery (including gene delivery), drug targeting, pharmaceutical technology, pharmaceutical biotechnology and clinical drug evaluation. The journal will typically not give priority to manuscripts focusing primarily on organic synthesis, natural products, adaptation of analytical approaches, or discussions pertaining to drug policy making.
Scientific commentaries and review articles are generally by invitation only or by consent of the Editors. Proceedings of scientific meetings may be published as special issues or supplements to the Journal.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


