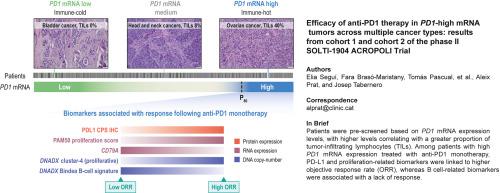
Efficacy of anti-PD1 therapy in PD1-high mRNA tumors across multiple cancer types: results from cohort 1 and cohort 2 of the phase II SOLTI-1904 ACROPOLI trial
IF 8.3
2区 医学
Q1 ONCOLOGY
引用次数: 0
Abstract
Background
Immune checkpoint inhibitors (ICIs) have transformed the treatment of solid tumors, yet responses vary across cancer types, highlighting the need for reliable pan-cancer biomarkers. Prior retrospective studies identified PD1 mRNA as a promising predictor of anti-PD1 response, but prospective validation was lacking.
Patients and methods
The SOLTI-1904 ACROPOLI trial was a non-randomized, open-label, multicenter phase II study designed to evaluate the efficacy of anti-PD1 monotherapy in patients with advanced solid tumors and high PD1 mRNA expression (cohort 1). An exploratory cohort also enrolled PD1-low tumors from cancer types with known ICI sensitivity (cohort 2). Between April 2021 and March 2022, 1003 patients across 33 cancer types were prescreened; 10.6% were classified as PD1-high. A total of 56 PD1-high and 15 PD1-low cases were enrolled to receive spartalizumab (400 mg intravenously every 4 weeks). The primary endpoint was objective response rate (ORR) in PD1-high tumors. Cohorts 1 and 2 were closed early due to discontinuation of spartalizumab development, before reaching full accrual.
Results
In the PD1-cohort, which included heavily pretreated tumors (median two prior lines) across 19 histologies, the ORR was 17.9% (95% confidence interval 7.8% to 27.9%). Among patients with tissue samples collected within 12 months of treatment, the ORR increased to 33.3%. The clinical benefit rate (partial response or stable disease ≥24 weeks) was 30.4%. Responses occurred in cancers typically resistant to ICIs, including pancreatic and microsatellite-stable colorectal cancers. No new safety signals were identified. Programmed death-ligand 1 expression by immunohistochemistry was significantly associated with response, whereas exploratory analyses identified additional potential biomarkers, including B cell gene signatures and tumor proliferation markers.
Conclusions
PD1 mRNA may help identify immunogenic tumors across cancer types. However, the trial’s early closure and exploratory nature warrant further validation. A composite biomarker strategy integrating immune and tumor-intrinsic features may improve patient selection for ICIs.
抗pd1治疗对多种癌症类型的pd1 -高mRNA肿瘤的疗效:来自SOLTI-1904 ACROPOLI II期试验的队列1和队列2的结果
背景:免疫检查点抑制剂(ICIs)已经改变了实体瘤的治疗方法,但不同癌症类型的反应不同,这突出了对可靠的泛癌症生物标志物的需求。先前的回顾性研究确定PD1 mRNA是抗PD1反应的有希望的预测因子,但缺乏前瞻性验证。患者和方法:SOLTI-1904 ACROPOLI试验是一项非随机、开放标签、多中心的II期研究,旨在评估抗PD1单药治疗晚期实体瘤和高PD1 mRNA表达患者的疗效(队列1)。一个探索性队列也纳入了来自已知ICI敏感性的癌症类型的低pd1肿瘤(队列2)。在2021年4月至2022年3月期间,对33种癌症类型的1003名患者进行了预筛选;10.6%为pd1高。共有56例pd1高患者和15例pd1低患者接受斯巴达单抗治疗(每4周静脉注射400mg)。主要终点是pd1高肿瘤的客观缓解率(ORR)。队列1和2由于停止斯巴达单抗开发而提前关闭,在达到完全累积之前。结果:在pd - 1队列中,包括19种组织学的大量预处理肿瘤(中位数为两条既往线),ORR为17.9%(95%置信区间为7.8%至27.9%)。在治疗12个月内收集组织样本的患者中,ORR增加到33.3%。临床获益率(部分缓解或病情稳定≥24周)为30.4%。反应发生在对ICIs具有典型抗性的癌症中,包括胰腺癌和微卫星稳定型结直肠癌。没有发现新的安全信号。免疫组织化学程序性死亡配体1的表达与应答显著相关,而探索性分析发现了其他潜在的生物标志物,包括B细胞基因特征和肿瘤增殖标志物。结论:PD1 mRNA可能有助于识别不同癌症类型的免疫原性肿瘤。然而,该试验的提前结束和探索性需要进一步验证。结合免疫和肿瘤固有特征的复合生物标志物策略可以改善患者对ICIs的选择。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ESMO Open
Medicine-Oncology
CiteScore
11.70
自引率
2.70%
发文量
255
审稿时长
10 weeks
期刊介绍:
ESMO Open is the online-only, open access journal of the European Society for Medical Oncology (ESMO). It is a peer-reviewed publication dedicated to sharing high-quality medical research and educational materials from various fields of oncology. The journal specifically focuses on showcasing innovative clinical and translational cancer research.
ESMO Open aims to publish a wide range of research articles covering all aspects of oncology, including experimental studies, translational research, diagnostic advancements, and therapeutic approaches. The content of the journal includes original research articles, insightful reviews, thought-provoking editorials, and correspondence. Moreover, the journal warmly welcomes the submission of phase I trials and meta-analyses. It also showcases reviews from significant ESMO conferences and meetings, as well as publishes important position statements on behalf of ESMO.
Overall, ESMO Open offers a platform for scientists, clinicians, and researchers in the field of oncology to share their valuable insights and contribute to advancing the understanding and treatment of cancer. The journal serves as a source of up-to-date information and fosters collaboration within the oncology community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


