Revealing probiotic properties of Lactiplantibacillus plantarum and Enterococcus faecalis in Cornu aspersum animal model
IF 2.4
3区 农林科学
Q1 FISHERIES
引用次数: 0
Abstract
This study explores the probiotic potential, immunomodulatory capacity, and safety of Lactiplantibacillus plantarum and Enterococcus faecalis strains isolated from the intestinal tract of the edible terrestrial snail Cornu aspersum maxima. Although host-microbe interactions are well studied in vertebrates, such research remains limited in invertebrates, particularly snails. To address this gap, 12 lactic acid bacteria strains were isolated and screened for tolerance to the defense mechanisms of snails and probiotic-associated traits, followed by machine learning (ML) predictions of immunomodulatory potential. According to results, 10 strains exhibited high tolerance to the external and internal defense mechanisms of snails (pedal and gastric mucus, gastric juices, low gut pH) in association with increased autoaggregation and hydrophobicity values and were predicted to have 100 % probability of eliciting immunomodulatory activity in vivo. Five strains, the L. plantarum Spp1 and Spp11 and E. faecalis Spp3, Spp8, Spp19, were selected for in vivo evaluation. Strain-specific immune responses were observed, with some strains mainly induced cellular immune responses, such as chemotaxis and phagocytic activity of hemocytes, while others also induced humoral responses. However, safety evaluations revealed that certain E. faecalis strains exhibited antimicrobial resistance or induced inflammatory reactions. Only two strains, the L. plantarum Spp11 and E. faecalis Spp19, were validated as safe and effective immunomodulatory probiotics in vivo. Overall, this study provides a comprehensive comparative analysis of the functionality of probiotic Lactiplantibacillus and Enterococcus strains in snails. These findings advance our understanding of snail-microbe symbiosis, particularly in the context of host-probiotic interactions, and support the use of C. aspersum as a valuable invertebrate model for probiotic research.
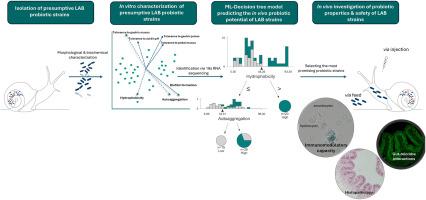
植物乳杆菌和粪肠球菌在牛角动物模型中的益生菌特性研究。
本研究旨在探讨从食用地蜗牛角牛肠道中分离的植物乳杆菌和粪肠球菌的益生菌潜力、免疫调节能力和安全性。尽管宿主-微生物的相互作用在脊椎动物中得到了很好的研究,但在无脊椎动物,特别是蜗牛方面的研究仍然有限。为了解决这一空白,分离了12株乳酸菌菌株,并筛选了对蜗牛防御机制和益生菌相关性状的耐受性,随后进行了机器学习(ML)预测免疫调节潜力。结果显示,10株菌株对蜗牛的外部和内部防御机制(足部和胃粘液、胃液、低肠道pH)表现出较高的耐受性,并伴有自身聚集和疏水性值的增加,预计在体内有100%的可能性激发免疫调节活性。选取植物乳杆菌Spp1、Spp11和粪乳杆菌Spp3、Spp8、Spp19 5株进行体内评价。观察到菌株特异性免疫反应,一些菌株主要诱导细胞免疫反应,如趋化性和血细胞吞噬活性,而另一些菌株也诱导体液免疫反应。然而,安全性评估显示,某些粪肠球菌菌株表现出抗微生物药物耐药性或诱导炎症反应。只有两株植物乳杆菌Spp11和粪乳杆菌Spp19在体内被证实是安全有效的免疫调节益生菌。总的来说,本研究提供了益生菌乳酸杆菌和肠球菌菌株在蜗牛体内功能的全面比较分析。这些发现促进了我们对蜗牛-微生物共生关系的理解,特别是在宿主-益生菌相互作用的背景下,并支持将C. aspersum作为益生菌研究的有价值的无脊椎动物模型。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
6.20
自引率
6.90%
发文量
206
审稿时长
49 days
期刊介绍:
Developmental and Comparative Immunology (DCI) is an international journal that publishes articles describing original research in all areas of immunology, including comparative aspects of immunity and the evolution and development of the immune system. Manuscripts describing studies of immune systems in both vertebrates and invertebrates are welcome. All levels of immunological investigations are appropriate: organismal, cellular, biochemical and molecular genetics, extending to such fields as aging of the immune system, interaction between the immune and neuroendocrine system and intestinal immunity.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


