Sulfidation-engineered magnetic LDHs/Biotite composite: Synergistic adsorption and magnetic recovery for heavy metal decontamination in soil
IF 4.3
2区 工程技术
Q2 ENGINEERING, CHEMICAL
引用次数: 0
Abstract
Soil contamination with heavy metals poses a serious threat to ecosystem safety and human health, highlighting the need for efficient adsorbents for remediation. A novel magnetic composite adsorbent was developed in this study through the in-situ growth of layered double hydroxides (LDHs) on biotite followed by sodium dimethyldithiocarbamate (DTC) intercalated (DTC-LDH/Biotite), designed for the efficient removal of Cu(II), Pb(II), As(V), and Cr(VI) from contaminated soil. The successful insertion was confirmed by characterization of C3H6NS2− into the interlayers of LDHs. A three-dimensional porous structure was revealed by SEM with uniformly dispersed LDHs on Biotite surface. Adsorption kinetics followed both the pseudo-second-order model and intraparticle diffusion model, indicating the adsorption process was predominantly controlled by active sites. Isotherm studies demonstrated that Cu(II)/Pb(II) adsorption followed the Freundlich model, indicative of multilayer sorption, while As(V)/Cr(VI) adsorption aligned with the Langmuir model, consistent with monolayer sorption. The maximum adsorption capacities reached 198.7 mg/g (Cu), 211.5 mg/g (Pb), 175.2 mg/g (As), and 163.8 mg/g (Cr). It was revealed by mechanistic analyses that Cu/Pb were immobilized through complexation with C3H6NS2− and with hydroxyl groups to form precipitates, whereas As/Cr were fixed through anion exchange and redox reactions. Soil column tests demonstrated that DTC-LDHs/Biotite effectively intercepted heavy metals within the 10–20 cm soil layer, minimizing vertical migration. The composite enabled efficient magnetic separation, with removal rates for As(V) and Cr(VI) remaining above 82 % after five cycles. This work presents a sustainable strategy for heavy metal remediation, integrating high adsorption efficiency, and recyclability, offering promising applications in soil pollution control.

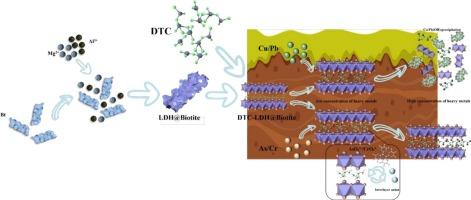
硫化工程磁性LDHs/黑云母复合材料:协同吸附和磁性回收土壤重金属净化
土壤重金属污染对生态系统安全和人类健康构成严重威胁,需要高效的吸附剂进行修复。本研究通过在黑云母上原位生长层状双氢氧化物(LDHs),然后插入二甲基二硫代氨基甲酸钠(DTC- ldh /黑云母),开发了一种新型磁性复合吸附剂,用于有效去除污染土壤中的Cu(II), Pb(II), As(V)和Cr(VI)。通过表征C3H6NS2−插入到LDHs的中间层中,证实了插入的成功。扫描电镜显示,黑云母表面具有均匀分散的ldh三维多孔结构。吸附动力学遵循伪二级模型和颗粒内扩散模型,表明吸附过程主要受活性位点控制。等温线研究表明,Cu(II)/Pb(II)的吸附符合Freundlich模型,属于多层吸附;As(V)/Cr(VI)的吸附符合Langmuir模型,属于单层吸附。最大吸附量分别为198.7 mg/g (Cu)、211.5 mg/g (Pb)、175.2 mg/g (As)和163.8 mg/g (Cr)。机理分析表明,Cu/Pb通过与C3H6NS2 -和羟基络合形成沉淀,而As/Cr通过阴离子交换和氧化还原反应固定。土柱试验表明,DTC-LDHs/黑云母能有效拦截10-20 cm土层内的重金属,使垂直迁移最小化。该复合材料实现了高效的磁分离,经过5次循环后,对As(V)和Cr(VI)的去除率保持在82% %以上。本研究提出了一种集高吸附效率和可回收性于一体的重金属可持续修复策略,在土壤污染控制中具有广阔的应用前景。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Chemical Engineering Science
工程技术-工程:化工
CiteScore
7.50
自引率
8.50%
发文量
1025
审稿时长
50 days
期刊介绍:
Chemical engineering enables the transformation of natural resources and energy into useful products for society. It draws on and applies natural sciences, mathematics and economics, and has developed fundamental engineering science that underpins the discipline.
Chemical Engineering Science (CES) has been publishing papers on the fundamentals of chemical engineering since 1951. CES is the platform where the most significant advances in the discipline have ever since been published. Chemical Engineering Science has accompanied and sustained chemical engineering through its development into the vibrant and broad scientific discipline it is today.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


