Cu–Ga Interactions and Support Effects in CO2 Hydrogenation to Methanol Catalyzed by Size-Controlled CuGa Nanoparticles Deposited on SiO2 and ZnO
IF 13.1
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
Growing environmental concerns have led to a need for the reduction of CO2 emissions and the search for alternative fuels. The synthesis of methanol via the CO2 hydrogenation reaction provides a promising approach for these tasks. Promoting the existing Cu-based catalysts with Ga might be an option to create more effective catalysts. Here, size-controlled bimetallic CuGa nanoparticles (NPs) supported on either SiO2 or ZnO were synthesized to study the nature of the interaction of Cu and Ga. Operando spectroscopy and diffraction characterization methods (XPS, XAS, XRD) were employed to establish structure, chemical composition, and reactivity correlations. We find that Ga stays oxidized under the reaction conditions and segregates to the surface. For the CuGa NPs/ZnO, the dominating interaction of Cu with ZnO inhibits the promoting effect of Ga. Only on the inert SiO2 support, the beneficial influence of Ga is visible. Furthermore, high pretreatment temperatures were found to result in a favorable Cu–Ga interaction by partially reducing Ga, which is beneficial for methanol selectivity.
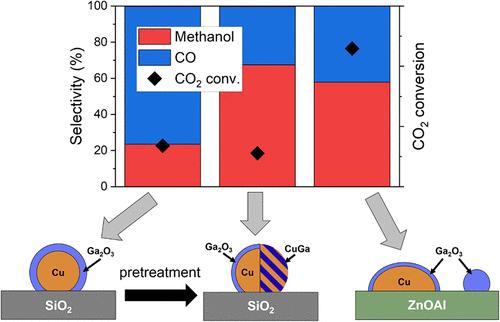
SiO2和ZnO上CuGa纳米颗粒粒径控制催化CO2加氢制甲醇的Cu-Ga相互作用及负载效应
日益增长的环境问题导致需要减少二氧化碳排放和寻找替代燃料。通过CO2加氢反应合成甲醇为这些任务提供了一种很有前途的方法。用Ga促进现有的cu基催化剂可能是创造更有效催化剂的一种选择。本文制备了尺寸可控的CuGa双金属纳米颗粒(NPs),分别负载在SiO2和ZnO上,研究了Cu和Ga相互作用的性质。利用Operando光谱和衍射表征方法(XPS, XAS, XRD)建立了结构,化学成分和反应性的相关性。我们发现Ga在反应条件下保持氧化状态,并向表面偏析。对于CuGa NPs/ZnO, Cu与ZnO的主导相互作用抑制了Ga的促进作用。只有在惰性SiO2载体上,Ga的有利影响才明显。此外,较高的预处理温度通过部分还原Ga而使Cu-Ga相互作用良好,有利于甲醇的选择性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Catalysis
CHEMISTRY, PHYSICAL-
CiteScore
20.80
自引率
6.20%
发文量
1253
审稿时长
1.5 months
期刊介绍:
ACS Catalysis is an esteemed journal that publishes original research in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. It offers broad coverage across diverse areas such as life sciences, organometallics and synthesis, photochemistry and electrochemistry, drug discovery and synthesis, materials science, environmental protection, polymer discovery and synthesis, and energy and fuels.
The scope of the journal is to showcase innovative work in various aspects of catalysis. This includes new reactions and novel synthetic approaches utilizing known catalysts, the discovery or modification of new catalysts, elucidation of catalytic mechanisms through cutting-edge investigations, practical enhancements of existing processes, as well as conceptual advances in the field. Contributions to ACS Catalysis can encompass both experimental and theoretical research focused on catalytic molecules, macromolecules, and materials that exhibit catalytic turnover.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


