Computational Investigation of the PazB-Catalyzed Cyclopropanation Reaction: Role of Active-Site Water in SN2 Mechanism
IF 13.1
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
We investigated the mechanism of PazB, a PLP-dependent enzyme involved in the biosynthesis of the cyclopropyl group in Pazamine, using DFT calculations, classical MD, and QM(GFN2-xTB)/MM/MD simulations. We found that two active-site water molecules selectively stabilize the rate-determining SN2 transition state over the reactant and product states by forming hydrogen bonds with the leaving chloride. Our studies reveal the structure and function of active site water molecules. Additionally, we found that PazB significantly enhanced the population of the reactive conformation, increasing its population from 29.6% in water to 92.4% in the enzyme’s active site.
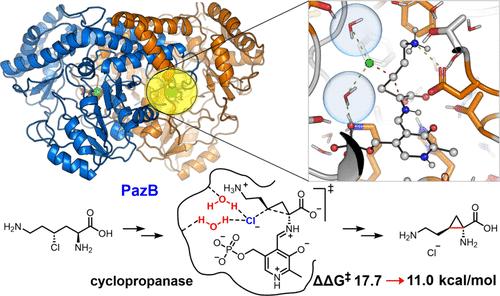
pazb催化环丙烷化反应的计算研究:活性位水在SN2机制中的作用
我们使用DFT计算、经典MD和QM(GFN2-xTB)/MM/MD模拟研究了PazB(一种plp依赖性酶)参与帕扎胺中环丙基的生物合成的机制。我们发现两个活性位置的水分子通过与离开的氯形成氢键,选择性地稳定了SN2在反应物和生成物状态上决定速率的过渡态。我们的研究揭示了活性位点水分子的结构和功能。此外,我们发现PazB显著提高了反应构象的种群数量,将其在水中的种群数量从29.6%增加到酶活性位点的92.4%。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Catalysis
CHEMISTRY, PHYSICAL-
CiteScore
20.80
自引率
6.20%
发文量
1253
审稿时长
1.5 months
期刊介绍:
ACS Catalysis is an esteemed journal that publishes original research in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. It offers broad coverage across diverse areas such as life sciences, organometallics and synthesis, photochemistry and electrochemistry, drug discovery and synthesis, materials science, environmental protection, polymer discovery and synthesis, and energy and fuels.
The scope of the journal is to showcase innovative work in various aspects of catalysis. This includes new reactions and novel synthetic approaches utilizing known catalysts, the discovery or modification of new catalysts, elucidation of catalytic mechanisms through cutting-edge investigations, practical enhancements of existing processes, as well as conceptual advances in the field. Contributions to ACS Catalysis can encompass both experimental and theoretical research focused on catalytic molecules, macromolecules, and materials that exhibit catalytic turnover.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


