Decarboxylative and Deformylative Alkyl–Alkyl Cross-Coupling Enabled by Nickel/Photoredox Catalysis
IF 13.1
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
Alkyl carboxylic acids and aldehydes are among the most fundamental functional groups in organic chemistry. Their combined use as coupling fragments offers new opportunities for the construction of valuable C(sp3)–C(sp3) bonds, yet it remains unexplored. Here, we report a radical C(sp3)–C(sp3) cross-coupling of redox-active esters derived from carboxylic acids with 4-alkyl-dihydropyridines derived from aldehydes and enabled by nickel/photoredox dual catalysis in a redox-neutral manner. This mild and practical radical-coupling protocol is applicable for forging C(sp3)–C(sp3) bonds across a wide range of substrates, including 1°–1°, 1°–2°, and 1°–3° couplings, thereby allowing for facile access to a wide array of C(sp3)-enriched motifs, including highly congested quaternary carbon centers. The synthetic utility of this methodology is further demonstrated through the streamlined synthesis of several known compounds as well as its application in building distinctive 1,2-heteroatom carbon frameworks and C(sp3)–glycosides.
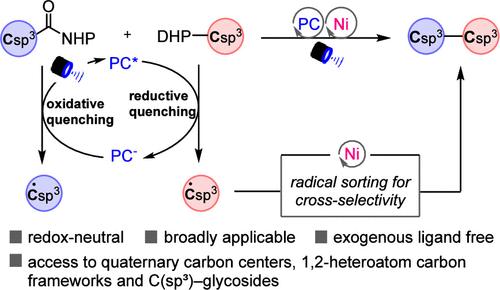
镍/光氧化还原催化实现脱羧和脱甲酰基烷基-烷基交叉偶联
烷基羧酸和醛是有机化学中最基本的官能团。它们作为耦合片段的组合使用为构建有价值的C(sp3) -C (sp3)键提供了新的机会,但它仍未被探索。在这里,我们报道了自由基C(sp3) -C (sp3)交叉偶联的氧化还原活性酯源自羧酸和4-烷基-二氢吡啶衍生的醛,实现了镍/光氧化还原双催化氧化还原中性的方式。这种温和而实用的自由基耦合协议适用于在广泛的衬底上锻造C(sp3) -C (sp3)键,包括1°-1°,1°-2°和1°-3°耦合,从而允许方便地访问各种富含C(sp3)的基元,包括高度拥挤的季碳中心。通过几种已知化合物的流线型合成以及在构建独特的1,2-杂原子碳框架和C(sp3) -糖苷方面的应用,进一步证明了该方法的合成效用。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Catalysis
CHEMISTRY, PHYSICAL-
CiteScore
20.80
自引率
6.20%
发文量
1253
审稿时长
1.5 months
期刊介绍:
ACS Catalysis is an esteemed journal that publishes original research in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. It offers broad coverage across diverse areas such as life sciences, organometallics and synthesis, photochemistry and electrochemistry, drug discovery and synthesis, materials science, environmental protection, polymer discovery and synthesis, and energy and fuels.
The scope of the journal is to showcase innovative work in various aspects of catalysis. This includes new reactions and novel synthetic approaches utilizing known catalysts, the discovery or modification of new catalysts, elucidation of catalytic mechanisms through cutting-edge investigations, practical enhancements of existing processes, as well as conceptual advances in the field. Contributions to ACS Catalysis can encompass both experimental and theoretical research focused on catalytic molecules, macromolecules, and materials that exhibit catalytic turnover.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


